Abstract
In overlapping distribution areas of Sorbus pohuashanensis and S. discolor in North China (Mount Tuoliang, Mount Xiling and Mount Baihua), Sorbus individuals were found with pink fruit, which have never been recorded for the flora of China. Fourteen morphological characters combined with four chloroplast DNA markers and internal transcribed spacer (ITS) were used to analyze the origin of the Sorbus individuals with pink fruits and their relationship to S. pohuashanensis and S. discolor. PCA, SDA and one-way (taxon) ANOVA of morphological characters provided convincing evidence of the hybrid origin of Sorbus individuals with pink fruits based on a novel morphological character and many intermediate characters. Haplotype analysis based on four cpDNA markers showed that either S. pohuashanensis or S. discolor were maternal parents of Sorbus individuals with pink fruits. Incongruence of the position of Sorbus individuals with pink fruits between cpDNA and ITS in cluster trees supported by DNA sequence comparative analysis, implying former hybridization events between S. pohuashanensis and S. discolor. Multiple hybridization events between S. pohuashanensis and S. discolor might have contributed to the generation of Sorbus individuals with pink fruits. This study has provided insights into hybridization between species of the same genus in sympatric areas, which is of great significance for the study of interspecific hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural hybridization plays a crucial role in evolution within the plant kingdom and has diverse evolutionary consequences, including the generation of genetic diversity and new ecotypes or species and the breakdown or reinforcement of isolation barriers between species (Emms and Arnold 1997; Coyne and Orr 2004; Wang 2017). Cross-fertilization between closely related taxa in the same region can result in hybrids (Jiggins and Mallet 2000; Rieseberg and Buerkle 2002), including among Sorbus L. (Rosaceae) taxa.
Morphological characters (primarily of leaves and fruits) have long been used to classify Sorbus hybrid taxa (Németh et al. 2020). Hybrids typically exhibit a mosaic of parent-like, intermediate and transgressive characters (Uhrinová et al. 2017; Yuan et al. 2019; Wang et al. 2021), though novel characters appear frequently in hybrid characters (Zhang et al. 2007). However, morphological characters of natural hybrids are influenced by the environment and expression of genes (Rieseberg et al. 1993) and cannot be used alone to detect putative hybrid individuals.
DNA sequence analysis is therefore commonly used to detect hybridization. Due to uniparental inheritance, chloroplast DNA (cpDNA) rarely recombines and can thus disclose information about current population structure and previous ancestry (Park et al. 2020; Van et al. 2021), gene flow (seed flow) between and within populations (Aizawa and Iwaizumi 2020), and the identity of the plastid-donor parent of the offspring (perhaps maternal in angiosperms) (Triplett and Clark 2021). The nuclear ribosomal internal transcribed spacer (nrITS) region is a universal species-specific marker for plants, which inherits from both parents and contains more information than the cpDNA marker. Accordingly, it is often used to determine the approximate range of the parents to avoid incorrect phylogenetic interpretations caused by uniparental genetic inference (Li et al. 2011; Nobre et al. 2018). Thus, the two types of genetic markers are usually integrated to identify the putative hybrid individuals, infer the maternal contributor, and provide insights into ancient hybridization (Vaezi et al. 2019; Yousefzadeh et al. 2019).
Approximately 100 species belong to Sorbus. They are widely distributed in the temperate zone of the Northern Hemisphere throughout Europe, Asia, and northern North America, and the main distribution centers are in the Himalayas, Caucasus, and China (McAllister 2005). From an evolutionary point of view, Sorbus is an ideal model for studying the consequences of natural interspecific hybridization, polyploidy, and apomixis, which drive the ongoing genetic diversity in Sorbus (Robertson et al. 2010; Ludwig et al. 2013; Németh et al. 2020). In Europe, many Sorbus hybrids have been found (Meyer et al. 2014; Sennikov et al. 2016; Hebda et al. 2021) and the resulting taxonomic complexity has made Sorbus one of the most taxonomically difficult groups of vascular plants and led to debate over concepts and delimitation of Sorbus species (Robertson and Sydes 2006). In China, there are 67 species of Sorbus (Yü and Lu 1974). S. aucuparia subsp. pohuashanensis (Hance) McAll. (2n = 2x = 34, subgenus Sorbus) and S. discolor (Maxim.) Maxim. (2n = 2x = 34, subgenus Albocarmesinae) are native tree species in China, which are distinct primarily in fruit color; S. aucuparia subsp. pohuashanensis (Hance) McAll. (hereafter called S. pohuashanensis) with red or orange fruits and S. discolor with white fruits. Both species grow in mixed broad-leaved forests in montane as an associated species at 500–2000 m a.s.l. in warm temperate zone. Pink-fruited Sorbus (hereafter called SH) individuals were found in Hebei Province (Mount Tuoliang, Mount Xiling) and Beijing (Mount Baihua), where the two native Sorbus species are commonly distributed. Because eFlora of China has no records of SH individuals, their origins and relationships to S. pohuashanensis and S. discolor are not clear. Sequence analysis of two cpDNA markers showed that either S. pohuashanensis or S. discolor was the female parent of SH individuals (Tang et al. 2016).
In this study, 14 morphological characters, combined with four noncoding cpDNA markers (matK, trnL-F, trnS-G, rpl16) and ITS were used to analyze the evolution of these SH individuals and determine whether they originated from the hybridization between S. pohuashanensis and S. discolor and, if so, how this event occurred. We aim to (1) investigate the morphological and molecular evidence for hybridization between S. pohuashanensis and S. discolor, (2) verify the maternal parent of the SH individuals and (3) explore possible pathways for the crossing.
Materials and methods
Sampling of plant material
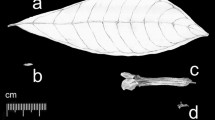
Several field surveys were conducted at Mount Tuoliang (HTL), Mount Xiling (HXL) and Mount Baihua (BBH) in North China, where S. pohuashanensis and S. discolor grow together with SH individuals at the same area (Fig. 1a). Mature, undamaged complete compound leaves and fruits without disease and insects were collected from individuals more than 10 m apart. Besides the collected individuals, the geographical coordinates of other Sorbus individuals for each site were determined using GPS. The altitudinal distribution pattern was analyzed after field surveys. A total of 85 individuals with fruits were included in morphological analyses, and the leaf material of 65 individuals was dried on silica gel and stored at − 80 °C for molecular analyses (Table 1).
In addition to fruit color, the presence of white hairs on the lower side of leaflets, winter buds, and young branches distinguished S. pohuashanensis from the glabrous S. discolor.
Morphological characters were measured and scored for 10–30 compound leaves and fruits sampled within the middle crown of individual trees from the three Sorbus groups at the three sites. For one leaflet chosen from the middle of each compound leaf, 12 quantitative characters were measured with 10–30 replications: length of compound leaf, width of compound leaf, ratio of length to width of compound leaf, length of leaflet, width of leaflet, ratio of length to width of leaflet, length of petiole, internal length between leaflets, pairs of leaflets, vertical length of fruit, horizontal length of fruit, ratio of vertical length/ horizontal length of fruit were measured and statistically analyzed after the field investigation. One-way (taxon group) ANOVA and the stepwise discriminant analysis (SDA) were carried out using SPASS 22 (Yockey and Pearson 2010). Principal component analysis (PCA) was conducted using the R package FactoMineR (Lê et al. 2008) implemented in R v. 3.6 (R Core Team 2020).
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from 20 mg of dried leaf tissue of each sample using Plant Genomic DNA Extraction Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions.
The primers used to amplify ITS and cpDNA are listed in Table 2 (Taberlet et al. 1991; Hamilton 1999; Parisod and Besnard 2007). The PCR reaction mixtures for ITS and cpDNA consisted of 20 µL containing 15 μL/mol DNA, 10 μL of 2 × Taq PCR Master Mix (Aidlab, Beijing, China), 0.25 mM of each primer (Majorbio, Shanghai, China), 7 μL of ddH2O. The thermocycling conditions (PCT-200, Bio-Rad, USA) were initial denaturation at 94 ℃ for 5 min; 30 cycles of 94 °C for 45 s, a suitable annealing temperature for each primer combination of between 52 and 55 °C for 45 s; then a final extension step at 72 °C for 10 min. PCR products were sequenced by the Majorbio Company (Shanghai, China) on an ABI 3730xl DNA Analyzer.
Molecular data analysis
Sequences were edited and assembled using BIOEDIT v7.0.5.3 (Hall 1999), and aligned using Clustal X (Thompson et al. 1997). Sequences of the outgroup species Pyrus pyrifolia (Burm. fil.) Nakai, Malus × micromalus Makino and Photinia serratifolia (Desf.) Kalkman were obtained from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/).
Chloroplast DNA haplotypes were identified by treating the four cpDNA markers as a whole without recombination, and nuclear haplotypes were identified using ITS sequences. Haplotype diversity (Hd) and nucleotide diversity (π) were calculated using DnaSP 5.0 (Librado and Rozas 2009). Haplotype networks for cpDNA and ITS were constructed separately using the software NETWORK V4.5.1.6 and the median-joining method (Bandelt et al. 1999) to ascertain the haplotype distribution in sampled populations and to identify putative hybridization events in particular.
ITS and the four combined cpDNA regions were phylogenetically analyzed using maximum-parsimony (MP), neighbor-joining (NJ), and Bayesian analysis. The best-fit model of molecular evolution required for Bayesian estimations was selected using the software MrModeltest 2.4 (Nylander 2004). GTR + I was the best model for ITS and GTR + I + G was the best for cpDNA. Bayesian posterior probabilities were estimated using MrBayes 3.2.7a (Ronquist et al. 2012). Bayesian analysis was started with random starting trees and maintained until the standard deviation of split sequences was below 0.05 as a convergence diagnostic value. Two independent Metropolis-coupled Markov chain Monte Carlo (MCMC) runs were done simultaneously, with one cold chain and three incrementally heated chains for each run; all runs were initiated with random starting trees and run for 10 × 105 generations for ITS and cpDNA. The fraction of the sampled values discarded as burn-in was set at 0.25. Neighbor-joining (NJ) and maximum-parsimony (MP) analyses were done using MEGA 7 (Kumar et al. 2016). The bootstrap analysis was performed to assess topological robustness with 1000 replicates using simple taxon addition (Felsenstein 1985). Bootstrap support values ≥ 70% were considered significant.
Results
Altitudinal distribution pattern of three Sorbus groups
SH individuals were found in HTL, HXL and BBH (Fig. 1a), which are the sympatric distribution areas of S. pohuashanensis and S. discolor. In HTL, S. discolor is mainly distributed at 1300–2000 m a.s.l., whereas S. pohuashanensis was mainly distributed at 1500–2200 m a.s.l. The SH individuals from HTL were distributed from 1500–1900 m a.s.l. (Fig. 1b), with S. discolor at the lower end of the range and S. pohuashanensis at the higher. S. pohuashanensis and S. discolor were distributed at 1386–1678 m a.s.l. at BBH and 1357–1578 m a.s.l. at HXL. At both sites, several SH individuals were found in areas with the two species.
Morphological evidence for hybridization
The SH individuals had pink fruit, a novel character that distinguishes it easily from the two local species. In terms of hairs on the surface of leaflet and young branch, SH individuals with few hairs were intermediate between S. pohuashanensis (dense) and S. discolor (glabrous). Regarding hairs on the outer surface of scales of winter bud, the SH individuals had sparse whitish-grey hairs only at the tip and margins, while S. pohuashanensis was densely and fully covered by hairs, and S. discolor had no hairs.
The ANOVA of the 12 quantitative morphological characters showed that nine characters (length of compound leaf, width of compound leaf, length of leaflet, width of leaflet, length of petiole, internal length between leaflets, pairs of leaflets and vertical length of fruit) had extremely significant differences among two Sorbus species and SH individuals (P < 0.01) (Table 3). The ratio of vertical length/horizontal length of fruit also differed significantly (P < 0.05). Most qualitative leaf and fruit characters of SH individuals were intermediate between S. pohuashanensis and S. discolor. The vertical length of fruit of SH individuals was significantly longer than that of S. pohuashanensis and S. discolor, but horizontal length of fruit among the two Sorbus species and SH individuals did not differ significantly.
The stepwise discriminant analysis showed that individuals of the three groups clustered into three overlapping groups (Fig. 2). The first group consisted of S. pohuashanensis and five SH individuals with a discriminant accuracy of 70%. The second group included 12 SH individuals, five S. pohuashanensis and three S. discolor (discriminant accuracy: 60%), and the third group consisted of S. discolor and two SH individuals (discriminant accuracy: 94.3%). Length of compound leaf, length of petiole and vertical length of fruit were the main characters that contributed to group separation. The majority of individuals of each Sorbus taxon surrounded their respective group centroid. In the discriminant function plot, S. pohuashanensis showed the most intraspecific variation. Morphological characters of SH individuals were closer to those of S. pohuashanensis than S. discolor. The Fisher discriminant functions for S. discolor, SH individuals and S. pohuashanensis were as follows:
where, x1, x2, and x3 represent length of compound leaf, length of petiole and vertical length of fruit, respectively.
Based on the principal component analysis (PCA) of 12 quantitative characters (Fig. 3), two principal components were extracted. The first principal component (PC1) explained 89.50% of the total variability and included length of compound leaf, width of compound leaf, length of leaflet and length of petiole. The second principal component (PC2) explained 7.10% of the total variation in morphological characters. There were also interspecific morphological variations in the two Sorbus species and SH individuals. SH individuals were intermediate between S. pohuashanensis and S. discolor.
First two principal component analysis of three Sorbus groups using 12 morphological traits. CLL, length of compound leaf; CLW, width of compound leaf; CLL/CLW, ratio of length/width of compound leaf; LL, length of leaflet; LW, width of leaflet; LL/LW, ratio of length/width of leaflet; LP, length of petiole; ILL, internal length between leaflets; PL, pairs of leaflets
Sequence analysis of cpDNA region
The length of the aligned cpDNA fragments (matK, trnL-F, trnS-G, rpl16) was 3465 bp with 21 polymorphic sites. The cpDNA sequences of all S. discolor were identical, while the cpDNA sequences of S. pohuashanensis and the SH individuals were diverse, and the variation was mainly concentrated in trnS-G (Table 4).
In total, seven haplotypes were identified from 65 individuals with a haplotype diversity of Hd = 0.771 (Table 5). Haplotype diversity varied across populations, ranging from 0.000 (SDHXL, SDBBH, SDHTL) to 1 (SHBBH). Nucleotide diversity was estimated within the species as a whole (π = 0.00087) and within populations, ranging from 0.0000 (SDHXL, SDBBH, SDHTL) to 0.00114 (SHBBH). The haplotype frequencies of H1–H7 are 15.4%, 6.2%, 6.2%, 23.0%, 9.2%, 38.5%, 1.5%, respectively.
All samples of S. discolor had haplotype H6, while S. pohuashanensis had five haplotypes (H1–H5) (Table 5). S. pohuashanensis from HXL and BBH had two haplotypes (H4 and H5). S. pohuashanensis from HTL was diverse with H1–H3. SH individuals (HTL2, 3, 7–10; BBH1; HXL5) shared H6 with S. discolor, and the other SH individuals shared H2 (HTL6, H4 [BBH2; HTL5; HXL3, 4], and H5 (HTL1; HXL1, 2) with S. pohuashanensis. In addition, one individual (SH [HTL] 4) had a unique haplotype (H7; Fig. 4a).
Chloroplast and nuclear ITS haplotype networks (a, c) and phylogenetic trees (b, d) for two Sorbus species and SH individuals. a, Chloroplast haplotype network. b, Phylogenetic tree for cpDNA sequences using Bayesian approach. c, ITS haplotype network. d, Phylogenetic tree for ITS using Bayesian approach. SD, S. discolor; SP, S. pohuashanensis; SH, SH individuals; BBH, Mount Baihua; HTL, Mount Tuoliang; HXL, Mount Xiling; MM, Malus × micromalus Makino; PP, P. pyrifolia; PS, P. serratifolia. Circle sizes in networks correspond to the haplotype frequency of haplotype; pie charts indicate proportions. Black rhombus in network represents missing haplotypes. Mutational steps of network are indicated by black bars. Dashed lines in network represent gaps and missing data. Number behind “location” (i.e., HTL) are the sample codes. Support values were estimated with posterior probabilities (in percentages). The “–1” and “–2” after the name of each represents the two homologous sequences of the ITS sequence split degenerate bases. Number behind “H” (i.e., H1) outside the phylogenetic tree indicate haplotypes
Both the MP tree and NJ tree based on chloroplast sequences were consistent with the Bayesian tree, so only the Bayesian consensus tree is displayed (Fig. 4b). Phylogenetic analysis showed that nine SH individuals, including SH (HTL) 1, 4–6, SH (BBH) 2, SH (HXL) 1–4 clustered with S. pohuashanensis, and the others clustered with S. discolor (Fig. 4b). The phylogenetic analysis also showed that H5 was most closely related to H4, and they formed a monophyletic group together with H1–H3. S. pohuashanensis and some of the SH individuals shared haplotypes H1–H5.
Analysis of ITS sequences of two Sorbus species and SH individuals
A total of 12 variable sites were found in the 585-bp ITS sequences (Table 6). No variations in ITS or cpDNA sequences were detected in samples of S. discolor, while there were abundant and diverse variations among individuals of S. pohuashanensis. There were three types of ITS sequences of S. pohuashanensis. The first type of ITS sequences of S. pohuashanensis (SP [BBH] 2, 7, 8; SP [HTL] 3–5, 7–9, 11, 13, 15–16; SP [HXL] 1) is homozygous, which are completely different from the sequence of S. discolor. The second type of ITS sequences of S. pohuashanensis (SP [BBH] 1; SP [HTL] 1, 2, 6, 10, 12, 14) is also homozygous, which had a mutation at locus 130 and the bases at the other 11 loci were completely different from the sequence of S. discolor. The third type is heterozygous with six genotypes: (1) SP (BBH) 3, 5, SP (HXL) 6; (2) SP (BBH) 4, SP (HXL) 2; (3) SP (BBH) 6; (4) SP (HXL) 3; (5) SP (HXL) 4 and; (6) SP (HXL) 5. The ITS sequences of some SH (BBH1; HTL2, 3, 5–10; HXL2, 4, 5) were as same as S. discolor, while the rest had heterozygous loci, which had four genotypes: (1) SH (BBH) 2 and SH (HTL) 1; (2) SH (HXL) 1; (3) SH (HXL) 3; (4) SH (HTL) 4. Those SH individuals showed chromatogram additivity compared with S. discolor and S. pohuashanensis at most nuclear loci, providing support for a natural hybridization hypothesis between S. discolor and S. pohuashanensis.
A total of 12 ITS haplotypes were identified from ITS (Table 7 and Fig. 4c). There was only one haplotype H1 in the samples from three sites of S. discolor, but seven haplotypes (H6–H12) in the samples of S. pohuashanensis. In addition to the unique haplotypes H2–H5, the SH individuals shared H1 with S. discolor and H6, H7 with S. pohuashanensis. In all populations, mean nucleotide diversity (π) was 0.01024 (from 0 to 0.00443) and haplotype diversity (Hd) was 0.673 (0 to 0.848) as inferred from ITS (Table 7).
In the Bayesian phylogenetic tree (Fig. 4d), S. pohuashanensis and S. discolor clustered into separate branches. The majority of the SH individuals clustered with S. discolor, but only SH (HTL) 4 with heterozygous ITS sequences clustered with S. pohuashanensis and S. discolor. Moreover, this maternal parent of the individual with unique haplotype H7 for cpDNA could not be detected.
Discussion
Morphological and molecular evidence for hybridization
SH individuals had novel pink fruit, and most quantitative leaf and fruit characters were intermediate between S. pohuashanensis and S. discolor, which is consistent with individuals that are products of hybridization featured with novel traits or intermediate morphological characters in other studies (Robertson et al. 2004; Price and Rich 2007; Hajrudinović et al. 2015; Zbiljić et al. 2021). For instance, the morphology of hybrid offsprings (natural and artificial) of Rhododendron decorum Franch. and R. delavayi Franch. was intermediate between the parental species (Zheng et al. 2017). Hybrids were shown to be mosaics of parental-like, intermediate, and transgressive phenotypes in the central part of the Western Carpathians (Ďurkovič et al. 2012). However, hybrid offsprings with parent-like morphological characters were not found using cpDNA and ITS markers in this study. Further studies using other molecular markers, such as cpDNA combined with SSR or SNP in parent-like individuals that have red- or white-fruited are needed.
In addition to the morphological evidence, our comparative analysis of DNA sequences demonstrated hybridization between S. discolor and S. pohuashanensis. Therefore, we infer that the SH individuals are likely hybrid offsprings of S. discolor and S. pohuashanensis.
Parental origin
Sequence analyses of four cpDNA markers showed that either S. pohuashanensis or S. discolor is the female parent of SH individuals, which have multiple origins rather than one origin. This result is consistent with those from two cpDNA fragments (Tang et al. 2016).
Multiple origins are common for Sorbus hybrids. Hybrids between S. aucuparia (diploid/sexual reproduction) and S. hybrida L. (tetraploid/facultative parthenogenesis) have several independent origins, and each population originated from multiple crosses between local parents (Levin et al. 2018). Similar results were also found for other hybrid species, such as Argyranthemum sundingii L. Borgen, Helianthus deserticola Heiser, Hippophae goniocarpa Y. S. Lian, X. L. Chen and K. Sun ex Swenson and Bartish (Brochmann et al. 2000; Gross et al. 2003; Jiang et al. 2014).
Except for SH individuals with the same haplotype as the parents, 6% of the SH individuals have a unique haplotype, perhaps as a result of natural variations of these hybrids of S. pohuashanensis and S. discolor. In addition, perhaps the small sample size did not fully cover all female parent donors for SH individuals.
Putative scenarios of hybridization and possible crossing pathways
SH individuals were found to show chromatogram additivity compared with S. discolor and S. pohuashanensis at most nuclear loci, implying natural hybridization between two parental species. The cpDNA analyses showed that 42% of the SH individuals share the chloroplast haplotype with S. discolor, while 53% of the SH individuals share the chloroplast haplotype with S. pohuashanensis, suggesting that S. discolor and S. pohuashanensis may be the maternal donors of SH individuals with reciprocal gene flow between them.
According to the comparative analysis of the cpDNA and ITS data set of the SH individuals, two crossing pathways, SP/SD and SD/SD, were inferred as follows:
(1) SP/SD crossing type: SH(HTL)1, 4–6; SH(BBH)2; SH(HXL)1–4. These individuals belong to clade S. pohuashanensis in the cpDNA phylogenetic tree and shared the cpDNA haplotype with S. pohuashanensis. Furthermore, they were nested within the S. discolor clade in the ITS phylogenetic tree and shared the ITS haplotype with S. discolor, confirming S. discolor as the male parent. Those SH individuals (SP/SD crossing type) with incongruence in their position in the cpDNA and ITS phylogenetic trees are assumed to be interspecific hybrids of S. pohuashanensis and S. discolor and have undergone previous hybridization events or backcrosses with their parents in these three sympatric locations. In this case, S. pohuashanensis served as the maternal donor and S. discolor as the pollen donor. Using phylogenetic incongruence of different genes is a common and preferred method to identify interspecific hybridization (Wang 2017), such as Ficus L. (Huang et al. 2019), Acer L. (Areces-Berazain et al. 2020), and Lachemilla Rydb. (Morales‐Briones et al. 2018).
(2) SD/SD crossing type: SH(HTL)2, 3, 7–10, SH(BBH)1, SH(HXL)5. CpDNA haplotype and ITS sequences of these SH individuals were identical to those of S. discolor. Therefore, the molecular evidence is not sufficient to prove that these SH individuals are hybrids, because their female parent is S. discolor, but their fruit is pink. So, we speculate that those individuals are hybrids produced by a cross between S. discolor (female parent) and S. pohuashanensis (male parent). These individuals may have originated from an ancient hybridization event, following by multiple backcrosses between hybrid offspring and S. discolor. This process might provide opportunities for SH individuals to be genetically “restored” to S. discolor. Another hypothesis is that those SH individuals were individuals of S. discolor, with characters that have not yet been discovered or documented. This hypothesis needs to be tested by expanding or deepening the investigation of S. discolor, especially for populations without gene introgression from other Sorbus species.
The formation of SH individuals might involve biparental origins and multiple hybridization events. Such reticular evolution also occurred for the subgenus Cerasus Mill. group in Jeju Island, Korea (Cho et al. 2014). As the maternal parent, Prunus serrulata Lindl./Prunus sargentii Rehder crossed with P. spachiana f. ascendens and produced the hybrid Prunus yedoensis Matsum., which is more similar to P. spachiana f. ascendens due to repeated backcrossing with P. spachiana f. ascendens. A reverse direction of hybridization events was also detected. The diversity and variation of Cerasus plants were attributed to backcrossing and bidirectional hybridization.
ITS sequences of all individuals of S. discolor are identical. Some individuals of S. pohuashanensis are heterozygous in ITS sequences, which may be caused by the genetic introgression of S. discolor into S. pohuashanensis. However, the amount and direction of gene flow need further studies.
Possible factors contributing to hybridization
Environmental factors and biological features create opportunities for hybridization between S. pohuashanensis and S. discolor. First, the overlapping geographical distributions and similar habitats permit spatial contact for hybridizations that occur for other plant genera such as Rhododendron L. (Zhang et al. 2007), Ostryopsis Decne. (Wang et al. 2021) and Quercus L. (Li et al. 2021). Second, the possibility of interspecific natural hybridization of two closely related species in sympatric regions may be enhanced by the partially or totally overlapping flowering periods and identical pollinators. Phenological observations revealed that the two Sorbus species flower in April and May and are commonly pollinated by ants and bees. More importantly, the mating system of S. pohuashanensis and S. discolor is mainly outcrossing (McAllister 2005; Zheng et al. 2009). The hybrids can bear fruits and are suspected to reproduce.
The cross-compatibility between the two subgenera was proved by Sorbus variety Pink-Ness (pink-fruited), which was bred by crossing S. discolor (subgenus Albocarmesinae) and S. aucuparia (subgenus Sorbus) in Europe. Moreover, S. × pekinensis was mentioned as a natural hybrid of S. pohuashanensis and S. discolor in the monograph by McAllister (2005) but was not described. It occurs in mountain areas west of Beijing, as do HTL, BBH and HXL. So, we infer that SH individuals are natural hybrids of S. pohuashanensis and S. discolor, namely S. × pekinensis (McAllister 2005).
At present, S. pohuashanensis is used for urban greening in Northeast China due to its adaptability to the climate at high altitudes. However, it is difficult to grow at low elevations in North China because leaves can be burned by high temperature in summer. We found that the hybrids had fewer sunburned leaves and were more resistant to high temperatures than S. pohuashanensis possibly due to the heterosis. Heterosis of hybrids between S. pohuashanensis and S. discolor may be one of the ways to cope with this difficulty.
Description of S. × pekinensis
Trees to 10 m tall, usually in moist habitats such as shady slopes, semi-shady slopes, and valleys. Branchlets brown or purplish brown to grayish brown, tomentose-villous when young, gradually glabrescent, with small grayish-white lenticels; buds conic-ovoid, reddish-brown, with whitish-grey, sparsely tomentose margins, apex acuminate. Ovate-lanceolate compound leaves are 16.49–20.33 cm long and 9.37–11.77 cm wide; petioles 3.16–4.10 cm; stipules broadly ovate or semi-orbicular, margin coarsely sharply serrate; leaflets 5–7 pairs, 4.9–6.14 cm long, 1.54–2.00 cm wide, at intervals of 1.50–1.98 cm, ovate, upper and lower surface with sparse pubescence, basal leaflets usually smaller than others, top 1/3 margin singly or doubly toothed; lateral veins 9–16 pairs, slightly arcuate-anastomosing at margin. Compound corymbs terminal, densely flowered, 8.3–12.6 cm in diameter. Flowers 0.9–1.3 cm in diameter. Petals are broadly ovate or subrotund, sparsely hirsute at the base of upper surface, 6.2–4.2 mm long, 2.9–3.1 mm wide; stigma 3–5 mm. Sepals triangular, acuminate or acute, both surfaces tomentose. Fruit pink, sometimes with red dots, 7.23–8.85 mm long, 6.64–8.60 mm wide. Flowering in April–May, fruiting in September–October.
Conclusions
Our morphological and molecular results provided evidence that Sorbus individuals with pink fruits are natural hybrids of S. pohuashanensis and S. discolor in three sympatric areas in North China. We verified that the maternal genome of the putative hybrid individuals came from either S. pohuashanensis or S. discolor. The results shed light on the formation and maintenance of genetic diversity in Sorbus and also provide an important basis for studying the mechanism and evolutionary process of interspecific hybridization between closely related species in sympatric areas. The results are important information for breeding Sorbus cultivars that can adapt to a warming climate. Because of the multiple copies of ITS sequences in nuclear DNA, the assumption of crossing origin of the putative hybrids needs to be verified by further cloning of ITS sequences. In addition, more molecular markers, such as simple sequence repeats or single-copy sequences of nuclear DNA should be deployed to confirm the natural hybridization events between the two Sorbus species in North China.
References
Aizawa M, Iwaizumi MG (2020) Natural hybridization and introgression of Abies firma and Abies homolepis along the altitudinal gradient and genetic insights into the origin of Abies umbellata. Plant Species Biol 35(2):147–157
Areces-Berazain F, Wang YX, Hinsinger DD, Strijk JS (2020) Plastome comparative genomics in maples resolves the infrageneric backbone relationships. PeerJ 8:e9483
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16(1):37–48
Brochmann C, Borgen L, Stabbetorp OE (2000) Multiple diploid hybrid speciation of the Canary Island endemic Argyranthemum sundingii (Asteraceae). Plant Syst Evol 220(1):77–92
Cho MS, Kim CS, Kim SH, Kim TO, Heo KI, Jun J, Kim SC (2014) Molecular and morphological data reveal hybrid origin of wild Prunus yedoensis (Rosaceae) from Jeju Island, Korea: implications for the origin of the flowering cherry. Am J Bot 101(11):1976–1986
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland
Ďurkovič J, Kardošová M, Čaňová I, Lagaňa R, Priwitzer T, Chorvát D Jr, Cicák A, Pichler V (2012) Leaf traits in parental and hybrid species of Sorbus (ROSACEAE). Am J Bot 99(9):1489–1500
Emms SK, Arnold ML (1997) The effect of habitat on parental and hybrid fitness: transplant experiments with Louisianan irises. Evolution 51:1112–1119
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125(1):1–15
Gross BL, Schwarzbach AE, Rieseberg LH (2003) Origin (s) of the diploid hybrid species Helianthus deserticola (Asteraceae). Am J Bot 90(12):1708–1719
Hajrudinović A, Frajman B, Schönswetter P, Silajdžić E, Siljak-Yakovlev S, Bogunić F (2015) Towards a better understanding of polyploid Sorbus (Rosaceae) from Bosnia and Herzegovina (Balkan Peninsula), including description of a novel, tetraploid apomictic species. Bot J Linn Soc 178(4):670–685
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol Ecol 8(3):521–523
Hebda A, Kempf M, Wachowiak W, Pluciński B, Kauzal P, Zwijacz-Kozica T (2021) Hybridization and introgression of native and foreign Sorbus tree species in unique environments of protected mountainous areas. AoB Plants 13(1):1–15
Huang JF, Xu R, Peng YQ (2019) Research progress of interspecific hybridization in genus ficus. Biodivers Sci 27(04):457–467 ((in Chinese))
Jiang YF, Yan R, Su X, Chen W, Sun K (2014) Molecular evidence for bidirectional hybrid origin and Hippophae rhamnoides ssp. sinensis as the mainly maternal plant of the diploid hybrid H. goniocarpa (Elaeagnaceae). Bull Bot Res 1:32–36 ((in Chinese))
Jiggins CD, Mallet J (2000) Bimodal hybrid zones and speciation. Trends Ecol Evol 15(6):250–255
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25(1):1–18
Levin J, Fay MF, Pellicer J, Hedrén M (2018) Multiple independent origins of intermediate species between Sorbus aucuparia and S. hybrida (Rosaceae) in the Baltic region. Nord J Bot, 36(12)
Li DZ, Gao LM, Li HT, Wang H, Ge XJ, Liu JQ, Chen ZD, Zhou SL, Chen SL, Yang JB, Fu CX, Zeng CX, Yan HF, Zhu YJ, Sun YS, Chen SY, Zhao L, Wang K, Yang T, Duan GW (2011) Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci U S A 108(49):19641–19646
Li X, Wei GM, El-Kassaby YA, Fang YW (2021) Hybridization and introgression in sympatric and allopatric populations of four oak species. BMC Plant Biol 21(1):1–14
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinform 25(11):1451–1452
Ludwig S, Robertson A, Rich TCG (2013) Breeding systems, hybridization and continuing evolution in Avon Gorge Sorbus. Ann Bot 111(4):563–575
McAllister H (2005) The genus Sorbus: mountain ash and other rowans. Royal Botanic Garden, Kew
Meyer N, Gregor T, Meierott L, Paule J (2014) Diploidy suggests hybrid origin and sexuality in Sorbus subgen. Tormaria from Thuringia Central Germany. Plant Syst Evol 300(10):2169–2175
Morales-Briones DF, Liston A, Tank DC (2018) Phylogenomic analyses reveal a deep history of hybridization and polyploidy in the Neotropical genus Lachemilla (Rosaceae). New Phytol 218(4):1668–1684
Németh C, Papp N, Nosková J, Höhn M (2020) Speciation by triparental hybridization in genus Sorbus (Rosaceae). Biol Futur 71(3):209–222
Nobre LLM, Santos JDO, Leite R, Almeida C (2018) Phylogenomic and single nucleotide polymorphism analyses revealed the hybrid origin of Spondias bahiensis (family anacardiaceae): de novo genome sequencing and comparative genomics. Genet Mol Biol 41(4):878–883
Nylander J (2004) Mrmodeltest v2. Uppsala University, Program distributed by the author, Evolutionary Biology Centre
Parisod C, Besnard G (2007) Glacial in situ survival in the Western Alps and polytopic autopolyploidy in Biscutella laevigata L. (Brassicaceae). Mol Ecol 16(13):2755–2767
Park JS, Jin DP, Choi BH (2020) Insights into genomic structure and evolutionary processes of coastal Suaeda species in East Asia using cpDNA, nDNA, and genome-wide SNPs. Sci Rep 10(1):1–11
Price DT, Rich TCG (2007) One-way introgressive hybridisation between Sorbus aria and S. torminalis (Rosaceae) in southern Britain. Watsonia 26(4):419–432
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Analysis, Vienna, Austria. Website https://www.R-project.org
Rieseberg LH, Buerkle CA (2002) Genetic Mapping in Hybrid Zones. Am Nat 159(S3):S36–S50
Rieseberg LH, Ellstrand NC, Arnold M (1993) What can morphological and molecular marker tell us about plant hybridization. CRC Crit Rev Plant Sci 12(3):213–241
Robertson A, Sydes C (2006) Sorbus pseudomeinichii a new endemic Sorbus (Rosaceae) microspecies from Arran. Scotland Watsonia 26(1):9–14
Robertson A, Newton AC, Ennos RA (2004) Multiple hybrid origins, genetic diversity and population genetic structure of two endemic Sorbus taxa on the Isle of Arran. Scotland Mol Ecol 13(1):123–134
Robertson A, Rich T, Allen AM, Houston L, Roberts CAT, Bridle JR, Harris SA, Hiscock SJ (2010) Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Mol Ecol 19(8):1675–1690
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542
Sennikov AN, Hjertson M, Salvesen PH (2016) Atlas Florae Europaeae notes 27. Taxonomy of the Sorbus arranensis group (Rosaceae) in Norway, a hybrid aggregate between S. aria s. lato and S. aucuparia. Ann Bot Fenn 53(1–2):1–13
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17(5):1105–1109
Tang W, Yu XD, Zhang CH, Ye XM, Fu QD, Zheng YQ (2016) Molecular evidence for maternal origin of Sorbus with pink fruits. For Res 29(6):834–838
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Triplett JK, Clark LG (2021) Hybridization in the temperate bamboos (Poaceae: Bambusoideae: Arundinarieae): a phylogenetic study using AFLPs and cpDNA sequence data. Syst Bot 46(1):48–69
Uhrinová V, Zozomová-Lihová J, Bernátová D, Paule J, Paule L, Gömöry D (2017) Origin and genetic differentiation of pink-flowered Sorbus hybrids in the Western Carpathians. Ann Bot 120(2):mcx013
Vaezi J, Arjmandi AA, Sharghi HR (2019) Origin of Rosa × binaloudensis (Rosaceae), a new natural hybrid species from Iran. Phytotaxa 411(1):23–38
Van DT, Xu B, Gao XF (2021) Molecular phylogeny and character evolution of Flemingia (Leguminosae, Papilionoideae, Phaseoleae, Cajaninae) inferred from three cpDNA and nrITS sequence data. Plant Syst Evol 307(2):1–17
Wang YG (2017) Natural hybridization and speciation. Biodivers Sci 25(6):565–576
Wang ZF, Jiang YZ, Bi H, Lu ZQ, Ma YZ, Yang XY, Chen NN, Tian B, Liu BB, Mao XX, Ma T, DiFazio SP, Hu QJ, Abbott RJ, Liu JQ (2021) Hybrid speciation via inheritance of alternate alleles of parental isolating genes. Mol Plant 14(2):208–222
Yockey RD, Pearson (2010) Spss demystified: a step by step approach. Pearson Schweiz Ag
Yousefzadeh H, Colagar AH, Yousefi E, Badbar M, Kozlowski G (2019) Phylogenetic relationship and genetic differentiation of Populus caspica and Populus alba using cpDNA and ITS noncoding sequences. J Forestry Res 30:451–461
Yü TT, Lu LD (1974) Sorbus L. Flora Reipublicae Popularis Sinicae, 36: 283–344. (in Chinese)
Yuan JL, Yue JJ, Zhong YB, Wu XL, Gu XP (2019) Genetic variation in distant and inbred hybridization progenies from three sympodial bamboo parent species. J Forestry Res 30(4):1323–1329
Zbiljić M, Lakušić B, Marčetić M, Bogdanović S, Lakušić D (2021) Morphological and chemical evidence of Teucrium×rohlenae K. Malý (Lamiaceae), a new hybrid in Croatia. Acta Bot Croat 80(1):48–55
Zhang JL, Zhang CQ, Gao LM, Yang JB, Li HT (2007) Natural hybridization origin of Rhododendron agastum (Ericaceae) in Yunnan, China: inferred from morphological and molecular evidence. J Plant Res 120(3):457–463
Zheng J, Zheng YQ, Zong YC, Li BJ (2009) Phenotypic diversity of fruits and seeds in natural populations of Sorbus pohuashanensis. Zhiwu Yichuan Ziyuan Xuebao 10(03):385–391 ((in Chinese))
Zheng SL, Tian XL, Huang CL, Wang LJ, Feng Y, Zhang JL (2017) Molecular and morphological evidence for natural hybridization between Rhododendron decorum and R. delavayi (Ericaceae). Biodivers Sci 5(6):627 ((in Chinese))
Acknowledgements
We thank Xuemin Ye and Qidi Fu of the Chinese Academy of Forestry for assistance during fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by the National Natural Science Foundation of China, Grant/Award Number: 32071779.
The online version is available at http://www.springerlink.com.
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Yu, X., Tang, W. et al. Morphological and molecular evidence for natural hybridization between Sorbus pohuashanensis and S. discolor (Rosaceae). J. For. Res. 35, 25 (2024). https://doi.org/10.1007/s11676-023-01659-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11676-023-01659-6