Abstract
The large larch beetle, Ips cembrae, is a significant pest causing the death of larch. In 2020, the attack density of I. cembrae on larch trap trees and standing trees was evaluated using sample sections placed along the trunk. As a defensive measure against I. cembrae, trap trees were highly effective in both spring and summer. The attack density increased with increasing trap tree surface area/volume. Galleries were established evenly throughout the entire trunk including the thin upper portion. When the number of trap trees was low and their capacity full, a continual aggregation of adults occurred due to pheromone communication, leading to attacks on healthy standing trees in the immediate vicinity. It was found that I. cembrae attacked standing trees from the trunk base, with a continual colonization of the stem up to 70% of the tree height in a time-differentiated progression of development stages. The attack density of I. cembrae on standing trees was up to 40% lower than on the trap trees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The large larch beetle, Ips cembrae (Heer), is a Euro-Siberian species of bark beetle (Scolytinae) (OEPP, EPPO 2005), which is the facultative primary pest attacking European larch stands (Larix decidua Mill.) in Europe. I. cembrae usually produces two generations annually depending on the weather (Krehan 2004) and altitude (Grodzki and Kosibowicz 2009). During outbreaks, the beetle attacks larch stands of all ages (Grodzki 2008; Grodzki and Kosibowicz 2009) at altitudes of 400–2400 m (Pfeffer and Knížek 1996; Grodzki 2020; Resnerová et al. 2020). Occasionally, it can also infest tree species of Abies, Picea and Pinus (Postner 1974). Local outbreaks have been recorded in Europe (Krehan and Cech 2004; Grodzki 2008, 2009; Grucmanová et al. 2014). Between 2000 and 2020, an average 467.8 ± 66.4 m3 of larch wood was harvested annually by salvage logging. Between 2005 and 2008, the portion of logged timber infested by I. cembrae increased by 0.5%; the most severe outbreak of I. cembrae occurred between 2015 and 2019 and the proportion of salvage logging increased up to 4.5% (Anonymous 2022).
I. cembrae infests the entire trunk with sporadic competition in narrow parts of the crown as well as bark beetles of the genera Pityophthorus, Pityogenes, and Cryphalus (Pfeffer 1955). After completing nuptial chambers, males copulate with usually three females. The galleries are three-armed in the trunk and two-armed in the crown (Arač and Pernek 2014). Maternal galleries are longer in the crown portion but the number of larval galleries is lower than in the lower part of the trunk (Holuša et al. 2014). The maturation feeding of beetles occurs either on the tree of their development or they leave the trunk and fly into the crowns of healthy trees, where they bore into fresh shoots (Knížek 2006).
The most effective way to control the density of bark beetle populations is to remove infested wood from the forest (Grégoire and Evans 2004), and the most effective defensive measure against I. cembrae, in addition to logging, is the use of trap trees (Holuša et al. 2021b). Trap trees are felled or uprooted healthy trees used to capture beetles and interrupt their development cycle (Grégoire and Evans 2004). For example, trap trees against I. typographus L., the European spruce bark beetle, have a limited capacity; they only capture a limited number of adults and require regular controls (Abgrall and Schvester 1987), which increases the time needed for this method (Bakke 1989). The emissions of aggregation pheromones produced by bark beetles in the trap tree and the filling of its capacity can lead to the infestation of healthy trees around the traps (Byers 1989), but if small bark beetle populations infest healthy trees, mass attack is prevented by host resistance (Sun et al. 2006).
Trap trees are installed in localities where I. cembrae occurred previously. According to Holuša et al. (2021b) as soon as a trap tree is fully colonized (0.4 entrance hole/dm2), it is necessary to start felling new trap trees. The aim of this study was: (1) to test the efficiency and capture effectiveness of trap trees against I. cembrae in comparation with attacked standing larch trees; and (2) to specify I. cembrae patterns of stem infestation and its impact on tree crowns.
The hypothesis is that the attack density on the trap tree will be higher than on living standing trees because these retain natural resistance (Rohde et al. 1996). I. cembrae has no competition with other bark beetles on larch and does not bore into specific stem parts unlike Ips typographus (L.), Ips amitinus (Eich.) and Ips duplicatus (Sahl.) on spruce or Ips sexdentatus (Börn.) and Ips acuminatus (Gyll.) on pine.
Materials and methods
Study sites
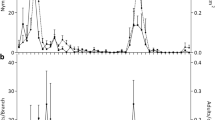
The study was carried out in 2020 at three study sites in the Děčín Forest District (Sněžník, Děčín Region, Czech Republic) (Fig. 1). In the 1970s and 1980s, the area was subject to industrial emissions and stands of Norway spruce died and were replaced by monocultures of L. decidua, Betula spp., Alnus spp. and Sorbus spp. The study sites, A, B and C were located in larch monocultures aged 35–41 years at altitudes of 460–600 m a.s.l., and with different rates of salvage logging of wood infested by I. cembrae from 2018 to 2020 (Table 1). The sites were selected according to early occurrence of I. cembrae infestation. Climatic data for 2020 were obtained from the Sněžník weather station (14°5′7.2954" E 50°47′48.5874" N; 569 m a.s.l.) (Fig. 2) (ČHMÚ 2022).
Location of study sites (Data source: Copernicus 2019)
Characteristics of traps and infested trees
Four larches for trap trees of the 1st series, to capture the offspring generation of I. cembrae, were felled 20–22 February 2020 in each location. Trap trees on the edge of stands were felled in the open, fully exposed to sun outside the stand. An average diameter at breast height (DBH) of the trap trees of the 1st series was 20.4 ± 3.6 cm and average length was 19.6 ± 2.0 m. Four trap trees of the 2nd series, to capture the summer generation of I. cembrae, were felled from 10 to 11 June 2020. Trees of the same DBH as the traps of the 1st series were felled for the 2nd trap tree series (DBH 17.5 ± 2.1 cm, average length 19.3 ± 1.2 m). To slow the process of phloem drying in the summer, the trap trees of the 2nd series were placed in semi-shade. The trap trees of the 1st and 2nd series were analyzed in May 27–31, 2020 and July 15–18, 2020.
To determine I. cembrae pattern of stem profile colonization, 36 standing, infested trees, site A = 16, Site B = 11, and Site C = 9 with average DBH of 22.0 ± 5.3 cm and lengths 20.0 ± 2.5 m were felled and analyzed for I. cembrae infestation in 20 June to 27 September 2020.
Modelling attack density on trap and infested trees
Four 0.5 m sections were identified on each trap tree and felled infested tree according to Grodzki (2004). The sections on the trap trees were named as T and on infested trees as I. The bottom section (I/T1) was at 0.8–1.5 m from the tree base. The stem-central section (I/T2) was between the base and the beginning of the crown, the sub-crown section (I/T3) was at the beginning of the crown, and the crown-central section (I/T4) was between the sub-crown and the top of the crown (Fig. 3).
Bark from the sample sections was removed from the circumference of stem. After beetle galleries in the phloem had been revealed, entry holes, maternal galleries, and the development stage (eggs, larvae, pupae, adults, emerged adults, dead beetles) were recorded. According to the number of entry holes and maternal galleries on the sections, the total number of males and females on the trap and infested trees was modelled. Based on the distance between the centre points of sample sections and the diameters of the sections, four parts were modelled, which together formed the total tree length. The surface area (dm2) and abundance (adults/dm2) on the model section were determined and then the total number of parental adults on the model section calculated. During the growing season, all larch trees up to 50 m from the trap trees were examined for infestation by I. cembrae. Infested trees were felled and analyzed the same way as the trap trees and the condition of phloem and crown recorded.
Data analysis
To compare the total numbers of I. cembrae adults in the trap trees and infested trees, only the totals up to Sect. 3 were compared because all trap trees (N = 24) were infested in 4 sections, whereas only 10 from 36 standing infested trees were infested. Up to Sect. 7, 18 infested trees were attacked. To compare the total capacity of trap and infested trees, the non-parametric Mann–Whitney U test was used.
To determine the attack density, eight categories were created according to the four sample sections on the traps (T) and infested (I) trees. The numbering of the sample sections (T1–4 and I1–4) depended on the position of the section on the stem. The total numbers of adults on the sections were compared using the non-parametric Kruskal–Wallis H test.
To compare the number of I. cembrae females on the sample sections, the sex index (number ♀♀ per ♂) was calculated from the number of maternal galleries and nuptial chambers (i.e., entrance holes). The total number of females were compared using the non-parametric Kruskal–Wallis H test.
During the growing season in 2020, infested trees were detected in all study sites and were classified according to visual crown condition as: healthy, fresh (H), fading (F) and dead (D) without needles. In each infested tree, the phloem was dying from the base up to the crown. The condition of the crowns and the proportion of dead phloem were compared using the non-parametric Kruskal–Wallis H test based on the proportion of the dead phloem as height of dead phloem/total tree length × 100. If the end of the dying phloem was not present in the section, the bark was removed up to the crown until the end of the dead phloem was found. Using Pearson’s correlation coefficient and general linear model (GLM), the relationship between the proportion of dead phloem and the height of the last gallery of I. cembrae was compared.
The pattern of infestation of trap and standing trees was evaluated using the constrained canonical-correlation analysis (CCA) in Canoco 5.0 software. The analysis included five stages of development of I. cembrae (eggs, larvae, pupae, adults, emerged adults) and were recorded as a proportion (0–100%) after debarking of the control section.
Results
Attack density of I. cembrae on trap and infested trees
A total of 186,748 I. cembrae adults bored into trap trees (N = 24). The I. cembrae numbers of the 1st and 2nd series (11.2 ± 4.2 and 9.6 ± 2.7 adults/dm2) did not significantly differ on the trap trees (W = 0.9313, p = 0.1041; t = 1.14, p = 0.3210). There was a significant difference in numbers on the trap trees at the study sites (W = 0.936, p = 0.134; F (2;21) = 4.519, p = 0.023) (Table 2).
The infested trees (N = 36) trapped 118,714 adults, although the number of trees was higher than the number of trap trees (N = 24). The total number was significantly higher on the trap trees (W = 0.917, p = 0.005; z = − 4.206, p = 0.00003) (Fig. 4). Pityogenes chalcographus L. was also found in the crowns of trap trees at site C.
Numbers of I. cembrae among the sections of trap trees and infested trees
The abundance of I. cembrae on the sections of the trap trees and infested trees differed significantly among the sections I1 × I4, T1, T2, T3; I2 × T1, T2, T3, T4; I3 × T1, T2, T3, T4; I4 × T1, T2, T3, T4 (W = 0.941, p = 0.00000; H (7, N = 240) = 139.755, p = 0.000).
While the number of entrance holes on the sections of the 1st and 2nd series of trap trees (T1–4) was similar (2.5 ± 1.0 and 2.0 ± 0.6 entrance holes/dm2, respectively), in the infested trees (I1–4) (0.9 ± 1.0 entrance holes/dm2), it decreased towards the crown to significantly lower values compared to the trap trees (Fig. 5). There were no differences in numbers of entrance holes on infested trees between study sites (W = 0.862, p = 0.0000; H (2, N = 144) = 4,752, p = 0.093).
Trap tree surface and number of captured adults
Using Pearson’s correlation coefficient, a positive correlation was found between the number of adults and the surface area of trap trees [r(48) = 0.427, p = 0.037]. The regression model was statistically significant and indicated that the number of captured adults increased with increasing surface area of the trap tree (R2 = 0.182, F = 4.904, p = 0.037) (Fig. 6).
Sex ratio of Ips cembrae in trap trees and infested trees
There was no significant difference between the numbers of females in the 1st and 2nd series of trap trees (W = 0.970, p = 0.026; z = –1.356, p = 0.175). The average sex ratios (♀♀: ♂) for the 1st and 2nd series were 3.4 ± 0.5 and 3.6 ± 0.4. No significant difference was found between the number of females on the trap trees and infested trees (W = 0.844, p = 0.0000; z = − 0.654, p = 0.513). The average sex ratio for the infested trees was 3.6 ± 0.9.
Tree crown condition and percentage of dead phloem
The average proportion of dead phloem over the total length was 65.8%. For trees with dead crowns, it was 93.4 ± 13.5%, trees with fading crowns 82.2 ± 17.1%, and for trees with healthy crowns it was only 37.6 ± 23.4% (Table 3). No fungal infections were present on the trees.
Larch needles were dying or fading if the phloem was dead to approximately 80% of total height (W = 0.878, p = 0.0009; H (2, N = 36) = 23.327, p = 0.0000). Trees with healthy crowns differed significantly from the other two categories in the proportion of dead phloem, but the categories of dead and fading crowns did not differ significantly (p = 1.0) (Fig. 7). There were no significant differences between the state of the crown and the height of the last gallery (W = 0.902, p = 0.0038; H (2, N = 36) = 4.184, p = 0.123).
In all trees, the phloem died from the base towards the crown. The last gallery was present above the dead phloem in 16 trees out of 36, with 12 of these having healthy crowns (H). There was a positive correlation between the height of the uppermost gallery and the proportion of dying phloem (r = 0.35, p = 0.038). The regression model was statistically significant and indicated that the proportion of dying phloem increased faster than the height of the last gallery (R2 = 0.32, F = 15.233, p = 0.0005) (Fig. 8).
Pattern of tree and trap tree infestation by Ips cembrae
The analysis of the control sections of live larch trees infested I. cembrae showed differences in the development stages along the stem. In infested trees with green needles and with no clear signs of dieback, I. cembrae galleries decreased from the base to the sub-crown section. An even more obvious dispersion was recorded on the trunks of dying trees (withering and falling needles). In the base, most of the galleries were empty as I. cembrae had completed its development. Eggs, larvae and pupae occurred only from the middle of the tree and culminated in the sub-crown section. On dead trees (without needles), galleries with completed development decreased from the base. Pupae occurred in the sub-crown section, and eggs and larvae in the crown (Table 4).
It was found that, on trees infested by I. cembrae, there were mostly galleries with development completed between the base and the mid trunk, and a new generation of adults and/or unfinished galleries due to the death of females. The middle part of the trunk was dominated by pupae, and larvae and eggs prevailed in the crown (CCA: p = 0.002) (Fig. 9).
In the trap trees of the 1st series, eggs, larvae, and pupae occurred evenly along the stem (adults and emerged galleries were not recorded, the trap trees were removed prior to the completion of I. cembrae development). On the trap trees of the 2nd series, larvae and pupae were dominant evenly in the entire stem. At the time of the analysis, eggs occurred sporadically, and emerging adults were recorded (low proportion before removing the trap trees) (Fig. 9).
The CCA analysis indicated that the large larch beetle attacked a standing tree from the base, gradually colonizing towards the crown, while the trap trees on the ground were infested concurrently over the entire stem, therefore the proportions of development stages are balanced.
Discussion
Trap trees cut from healthy larch before the swarming of I. cembrae is a standard control and defensive measure (Holuša et al. 2021a), similar to use of trap trees for spruce bark beetles, Ips typographus (L.), Ips amitinus (Eichh.), and Polygraphus poligraphus (L.) (Pfeil 1827; Holuša et al. 2021b), or pine traps for Tomicus spp., Ips acuminatus (Gyll.), Ips sexdentatus (Börner), and Hylastes spp. (Besceli and Ekici 1969; Zahradník 2014; Meurisse et al. 2021). Trap tree efficiency captures a wider range of bark beetle species than species-specific synthetic pheromones (e.g., Cembräwit for I. cembrae). However, unlike slot traps and poison trap trees with pheromone lures, trap trees have a limited capacity (Abgrall and Schvester 1987). According to Holuša et al. (2021a), the threshold capacity of larch trap tree for the large larch beetle is a 0.4 entrance hole/dm2.
The balanced and simultaneous infestation along the entire stem of the two series of trap trees confirmed their high efficiency in capturing I. cembrae, and is not limited by phloem thickness as is the case for other bark beetle species (Hutchison and Reid 2022). Our analysis of trap trees and standing infested trees indicates that the abundance of I. cembrae increased with increasing surface area of the trap tree. Resnerová et al. (2020) noted that bark beetle numbers decreased with increasing volume of the stem. The reason for the discrepancy may be because their three-year study included a larger number of sites with a differentiated population density of I. cembrae and different sizes of trap trees.
It appears that the number of entrance holes (2.3 ± 0.7/dm2) exceeded the capacity of trap trees during the study. The increased population density and natural pheromone communication signaling trap tree infestation (Symonds and Gitau-Clarke 2016), created conditions for I. cembrae attack on trees in the immediate vicinity of the trap trees (Byers 1989). In our study, infested trees occurred at an average distance of 19.8 ± 9.4 m from the nearest trap tree. It was found that during the outbreak of I. typographus, more than 80% of infested trees were within 100 m from the center of bark beetle outbreak. The highest probability of infestation is around uprooted trees and stem breaks. However, when the population density is high, colonization of healthy trees is scattered, with the probability of infestation decreasing sharply with increasing distance from the point of emergence (Wichmann and Ravn 2001).
The efficiency of the number of adults captured on the trap trees is affected by beetle population density, by trap tree insolation/shading (Holuša et al. 2021a), and by diameter at breast height. Arač and Pernek (2014) found the most common attack was on standing larch trees with 12.5–27.5 cm DBH. According to Resnerová et al. (2020), trap trees 0.25–1.5 m3 in volume can capture a comparable number of I. cembrae adults.
According to our results, the number of galleries by I. cembrae on the standing trees was significantly lower than on the trap trees. The reason may be the natural resistance of larch which releases resin into galleries, as well as phloem chemistry changes (Terasaki et al. 1987; Jung et al. 1994; Rohde 1995; Rohde et al. 1996). Mulock and Christiansen (1986) found that the TSA (Threshold for Successful Attack) of I. typographus adults on spruce increased with tree vigor. The mortality of females in the beginning of oviposition was particularly high in the base. The defensive process of trees can be disturbed by drought stress. When trees are stressed, emissions of volatiles and aggregation of bark beetles are greater (Redfern et al. 1987; Grodzki 2008; McDowell et al. 2008).
Synusia of bark beetles on trees has been described by Pfeffer (1955). Bark beetles within the stem are influenced by the place where the infestation began (Kula and Zabecki 2010). Ips typographus infests the stem in the lower part of the crown (Zumr 1984), while Ips amitinus attacks the mid crown first (Zumr 1984; Grodzki 1997; Kula and Zabecki 2001; Holuša et al. 2012) and Ips duplicatus infests the entire trunk of spruce but it also infests stems of spruce younger than 60 years of age (Grodzki 2012). With Pinus sylvestris, the infestation by the genus Ips is in the lower part of the trunk (I. sexdentatus) and to the crown (I. acuminatus) (Pfeffer 1955).
I. cembrae infests the entire stem of larch of any diameter with no interspecific competition. In the trap trees for I. cembrae, the entire trunk was infested evenly and numbers over all parts of the tree were balanced, which agrees with Holuša et al. (2014). This shows that trap trees were effectively capturing both spring and summer generations of I. cembrae over their entire length,whereas the numbers of I. cembrae on infested trees was statistically significantly decreasing from the base of the trunk to the crown. The analysis of the development stages of I. cembrae on the trees in various phases of trunk infestation and stage of crown dieback indicated the stem base as the place of initial attack from where the whole tree is continually infested. This explains the completed development of I. cembrae in the butt part of the stem, the dominance of larvae in the middle stem, and eggs with emerging larvae in the crown on trees with healthy crowns and fading crowns. Infested trees can be detected easily by the occurrence of frass. Detection by crown condition is not possible because the population in the lower part of the trunk has already completed its development when crown dieback occurs. The reason for infestation from the base of the stem may be because of a strategic weakening of the tree which is later predisposed for the following attack. There were no significant differences in the numbers of males and females in the different sections of trap trees and infested trees. The female/male ratio on infested trees is similar to data by Arač and Pernek (2014).
Conclusions
During a one-year experiment in 2020, the capture of I. cembrae by trap trees of the 1st series was higher than the 2nd series. Infestation of trap trees was significantly higher than on infested standing trees due to defensive reactions of live trees causing the mortality of males during the attack as well as of females making egg galleries.
The abundance of males of I. cembrae on the trap trees was balanced over the entire stem, whereas in the infested standing trees, the numbers decreased in sub-crown and crown.
The capture effectiveness of trap trees was positively affected by increasing volume and surface area of the trap tree.
The different development stages of the stem of infested standing trees indicated that I. cembrae attacks larch trees from the butt end, gradually colonizing towards the crown, while trap trees are invaded simultaneously over the entire stem. The simultaneous invasion of the whole stem of the two series of trap trees confirmed that trap trees are suitable for pest management of I. cembrae in the protection of larch stands.
References
Abgrall JF, Schvester D (1987) Observations on the trapping of Ips typographus L. after windthrow. Revue Forestière Française 39:359–377. https://doi.org/10.4267/2042/25808
Anonymous (2022) Zpravodaj ochrany lesa. Supplementum. [Text article], Retrieved from https://www.vulhm.cz/aktivity/vydavatelska-cinnost/zpravodaj-ochrany-lesa-supplementum/ [accessed on 20.01.2023].
Arač K, Pernek M (2014) Occurrence and spreading of the large larch bark beetle (Ips cembrae) in Croatia and possiblities of monitoring by using pheromone traps. Šumar List 138:145–154
Bakke A (1989) The recent (Ips typographus) outbreak in Norway—experiences from a control 719 program. Holartic Ecol 12:515–519. https://doi.org/10.1111/j.1600-0587.1989.tb00930.x
Besceli O, Ekici M (1969) Control and biology of Ips sexdentatus in the Picea orientalis region. Orm Arast Enst Tek Bult 32:1–32
Byers JA (1989) Behavioral mechanisms involved in reducing competition in bark beetles. Ecography 12(4):466–476. https://doi.org/10.1111/j.1600-0587.1989.tb00924.x
ČHMÚ (2022) Daily precipitation and average temperature in weather station Sněžník in year 2020. . https://www.chmi.cz/historicka-data/pocasi/denni-data/Denni-data-dle-z.-123-1998-Sb [accessed on 20.01.2023].
Copernicus (2019) Land Monitoring Service, Corine Land Cover (CLC) 2018, Version 20. . https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 [accessed on 20.01.2023].
Grégoire JC, Evans HF (2004) Damage and Control of BAWBILT Organisms—an Overview. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Springer, Dordrecht, pp 19–37
Grodzki W (1997) Pityogenes chalcographus (Coleoptera, Scolytidae) –an indicator of man-made changes in Norway spruce stands. Biologia 52(2):217–220
Grodzki W (2004) Some reactions of Ips typographus (L.) (Col.: Scolytidae) to changing breeding conditions in a forest decline area in the Sudeten Mountains. Poland. J Pest Sci 77(1):43–48. https://doi.org/10.1007/s10340-003-0026-1
Grodzki W (2008) Ips cembrae Heer. (Col.: Curculionidae, Scolytinae) in young larch stands – a new problem in Poland. Forstsch Aktuell 44:8–9
Grodzki W (2009) The larch bark beetle Ips cembrae (Heer) (Coleoptera, Curculionidae, Scolytinae) in young and older larch stands of southern Poland. Les Pr Bad 70(4):355–361. https://doi.org/10.2478/v10111-009-0033-y
Grodzki W (2012) Two types of Norway spruce Picea abies (L.) H. Karst. infestation by the double spined bark beetle Ips duplicatus C. R. Sahlb. (Coleoptera: Scolytinae) in southern and north-eastern Poland. Folia For Pol Ser A 54(3):169–174
Grodzki W (2020) On the vertical distribution of Ips duplicatus, I. cembrae and some bark- and longhorn beetles (Col.: Curculionidae, Scolytinae, Col.: Cerambycidae) in the Tatra National Park in Poland. Folia For Pol Ser A 62(2):68–77. https://doi.org/10.2478/ffp-2020-0008
Grodzki W, Kosibowicz M (2009) Contribution to the recognition of the biology of Ips cembrae (Heer) (Col., Curculionidae, Scolytinae) in the conditions of southern Poland. Sylwan 153(9):587–593
Grucmanová Š, Holuša J, Trombik J, Lukášová K (2014) Large larch bark beetle Ips cembrae (Coleoptera: Curculionidae, Scolytinae) in the Czech Republic: analysis of population development and catches in pheromone traps. For J 60:143–149. https://doi.org/10.2478/forj-2014-0015
Holuša J, Lukášová K, Grodzki W, Kula E, Matoušek P (2012) Is Ips amitinus (Coleoptera: Curculionidae) abundant in wide range of altitudes? Acta Zool Bulgar 64(3):219–228
Holuša J, Kula E, Wewiora F, Lukášová K (2014) Flight activity, within the trap tree abundance and overwintering of the larch bark beetle (Ips cembrae) in Czech Republic. Šumarski List 138:19–27
Holuša J, Resnerová K, Berčák R, Koreň M, Kula E (2021) Optimization of felled trap trees for bark beetles control. Lesnický průvodce, VÚLHM, p 42
Holuša J, Resnerová K, Kula E (2021) Application of the principles of integrated pest management against large larch bark beetle (Ips cembrae (Heer, 1836)). Lesnický průvodce, VÚLHM, p 62
Hutchison IK, Reid ML (2022) Phloem and defence traits in relation to tree size and age: implications for host selection by bark beetles. Forest Ecol Manag. https://doi.org/10.1016/j.foreco.2022.120183
Jung P, Rohde M, Lunderstadt J (1994) Induzierte Resistenz im Leitgewebe der Europaischen Larche Larix decidua Mill. nach Befall durch den Grossen Larchenborkenkafer Ips cembrae Heer (Col.: Scol.). J Appl Entomol 117:427–433. https://doi.org/10.1111/j.1439-0418.1994.tb00758.x
Knížek M (2006) Large larch bark beetle (Ips cembrae). Lesnická Práce 85:1–4
Krehan H (2004) Data sheet: Ips cembrae (Großer Lärchenborkenkäfer). Forstsch Aktuell 32:9
Krehan H, Cech TL (2004) Larch damage in Upper Styria. An example of the complex effects of damage agents. Forstsch Aktuell 32:4–8
Kula E, Ząbecki W (2001) Attractiveness of spruce for cambioxylophages as related to stand age. J for Sci 47:88–96
Kula E, Ząbecki W (2010) Merocoenoses of cambioxylophagous insect fauna of Norway spruce (Picea abies/L./Karst.) with focus on bark beetles (Coleoptera: Scolytidae) and types of tree damage in different gradation conditions. J For Sci 56:474–484. https://doi.org/10.17221/33/2010-jfs
McDowell N, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Plaut J, Sperry J, West A, William D, Yepez E (2008) Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol 178:719–739. https://doi.org/10.1111/j.1469-8137.2008.02436.x
Meurisse N, Pawson SM, Somchit C (2021) Bark beetles on pine logs: forecasting winter colonisation dynamics based on trap catches and temperature records. J Pest Sci 94(4):1357–1373. https://doi.org/10.1007/s10340-021-01361-8
Mulock P, Christiansen E (1986) The threshold of successful attack by Ips typographus on Picea abies: A field experiment. Forest Ecol Manag 14(2):125–132. https://doi.org/10.1016/0378-1127(86)90097-6
OEPP, EPPO (2005) Ips cembrae and Ips subelongatus. EPPO Bulletin 35:445–449. https://doi.org/10.1111/j.1365-2338.2005.00880.x
Pfeffer A, Knížek M (1996) Coleoptera: Curculionoidea 2 (Scolytidae and Platypodidae). Folia Fac Sci Nat Univ Masaryk Brun Biol 94:601–607
Pfeffer A (1955) Fauna of Czechoslovakia. Bark beetles-Scolytoidea. ČSAV, 324 pp.
Pfeil W (1827) About insect damage in forests, means of preventing it and reducing its damage. Verlag, Germany, p 72
Postner M (1974) Ips cembrae. In: Schwenke W (ed) Die Forstschädlinge Europas. Paul Parey, Germany, pp 458–459
Redfern DB, Stoakley JT, Steele H, Minter DW (1987) Dieback and death of larch caused by Ceratocystis laricicola sp. nov. following attack by Ips cembrae. Plant Pathol 36:467–480. https://doi.org/10.1111/j.1365-3059.1987.tb02264.x
Resnerová K, Holuša J, Surový P, Trombik J, Kula E (2020) Comparison of Ips cembrae (Coleoptera: Curculionidae) capture methods: small trap trees caught the most beetles. Forests 11:1275. https://doi.org/10.3390/f11121275
Rohde M (1995) Physiological investigations into susceptibility, defense reactions and resistance, in relations between larch and the larch bark beetle (Ips cembrae). M D Gesell Allg Ange 10:51–54
Rohde M, Waldmann R, Lunderstandt J (1996) Induced defence reaction in the phloem of spruce (Picea abies) and larch (Larix decidua) after attack by Ips typographus and Ips cembrae. Forest Ecol Manag 86:51–59. https://doi.org/10.1016/s0378-1127(96)03802-9
Sun XL, Wang QY, Sweeney JD, Gao CQ (2006) A review: chemical ecology of Ips typographus (Coleoptera, Scolytidae). J Forestry Res 17(1):65–75
Symonds MRE, Gitau-Clarke CW (2016) The evolution of aggregation pheromone diversity in bark beetles. Adv Insect Physiol 50:195–234. https://doi.org/10.1016/bs.aiip.2015.12.003
Terasaki Y, Yosida N, Fukuyama K, Furuta K (1987) Response of Larix leptolepis to inoculated Ips cembrae. Bul of the Tokyo Univ Forests 77:19–30
Viewegh J (2003) Czech forest (site) ecosystem classification. J For Sci. 49(2):74–82
Wichmann L, Ravn HP (2001) The spread of Ips typographus (L.) (Coleoptera, Scolytidae) attacks following heavy windthrow in Denmark, analysed using GIS. Forest Ecol Manag 148(1–3):31–39. https://doi.org/10.1016/S0378-1127(00)00477-1
Zahradník P (2014) Methodical manual of integrated plant protection for forest stands. Lesnická práce, 376
Zumr V (1984) Spatial distribution of bark beetles (Coleoptera, Scolytidae) in Norway spruce (Picea excelsa Link) and their indifference in relation to forest belts. Lesnictví 30:509–523
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by Ministry of Agriculture of the Czech Republic in grant [QK1920433]. “Influence of protective measures against the populations of bark beetles according to population density”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by Ministry of Agriculture of the Czech Republic in grant [QK1920433]. “Influence of protective measures against the populations of bark beetles according to population density”.
The online version is available at http://www.springerlink.co
Corresponding editor: Tao Xu
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Špoula, J., Kula, E. Patterns of Ips cembrae (Herr) infestation on trap and standing trees of European larch (Larix decidua Mill.). J. For. Res. 34, 2041–2050 (2023). https://doi.org/10.1007/s11676-023-01627-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-023-01627-0