Abstract
Climate change can intensify drought in many regions of the world and lead to more frequent drought events or altered cycles of soil water status. Therefore, it is important to enhance the tolerance to drought and thus health, vigor, and success of transplantation seedlings used in the forestry by modifying fertilization and promoting mycorrhization. Here, we sowed seeds of Japanese larch (Larix kaempferi) in 0.2-L containers with 0.5 g (low fertilization; LF) or 2 g (high fertilization; HF) of slow-release fertilizer early in the growing season. One month later, we irrigated seedlings with non-sterilized ectomycorrhizal inoculum (ECM) or sterilized solution (non-ECM), and after about 2 months, plants were either kept well watered (WW; 500 mL water/plant/week) or subjected to drought (DR; 50 mL water per plant/week) until the end of the growing season. HF largely stimulated plant growth and above- and belowground biomass production, effects that are of practical significance, but caused a small decrease in stomatal conductance (Gs390) and transpiration rate (E390), which in practice is insignificant. ECM treatment resulted in moderate inhibition of seedling growth and biomass and largely canceled out the enhancement of biomass and foliar K content by HF. DR caused a large decrease in CO2 assimilation, and enhanced stomatal closure and induced senescence. DR also largely depleted foliar Mg and enriched foliar K. Although DR caused a large decrease in foliar P content in LF, it moderately increased P in HF. Likewise, DR increased foliar K in HF but not in LF, and decreased foliar P in ECM plants but not in non-ECM plants. Conversely, ECM plants exhibited a large enrichment in foliar P under WW and had a lower water potential under DR when grown in LF. These results indicate that the drought tolerance and health and vigor of Japanese larch seedlings can be modified by soil fertility and soil microorganisms. This study provides a basis for new multifactorial research programs aimed at producing seedlings of superior quality for forestation under climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Larch trees have been widely planted and harvested in Japan, China, and other regions throughout the Eurasian continent, which are deciduous conifers promising as an eco-environmental resource (Chen et al. 2018; Qu et al. 2022). Japanese larch [Larix kaempferi (Lamb.) Carr; syn. L. leptolepis Sieb and Zucc.] is among the fastest-growing larch species, an eco-environmentally relevant trait that promoted its export to Europe as a pollen resource (Matyssek and Schulze 1987; Qu et al. 2022), and plays a vital role in silviculture in different regions of the world (Kochenderfer et al. 1995; Kurinobu 2015). Due to its desired characteristics for wood production, Japanese larch is a major plantation species in Japan and has been a target of breeding programs since the early 1950s (Kurinobu 2015). Moreover, it was promoted in other regions, such as on hardwood sites of poor quality as an alternative to white pine in Pennsylvania, United States (Kochenderfer et al. 1995). Nevertheless, recent studies indicate that Japanese larch does not perform well under nutrient imbalances and other environmental challenges that cause oxidative stress, raising concerns that desired wood characteristics would also decline (Li et al. 2018; Wang et al. 2018; Agathokleous et al. 2020).
Drought is considered the most-damaging natural disaster with the greatest potential to cause water and food crises on local to global scales, as exemplified by drought-induced famine in Africa and various ecological impacts in Asia (Hao et al. 2014). Importantly, increased aridity has been projected to affect wide regions worldwide during the twenty-first century (Dai 2011; Vicente-Serrano et al. 2020; Zhuang et al. 2022). Although ecosystems often exhibit resilience and resistance to drought events, usually with temporary effects, forest-related studies derived from inventories or tree ring chronologies suggest that drought is having greater effects on ecosystems (Vicente-Serrano et al. 2020).
Drought induces water deficit in plants, which causes oxidative stress and the generation of reactive oxygen species, which accelerate cell death and senescence and inhibit photosynthesis and growth in some species (Chaves et al. 2002; Lisar et al. 2012; Petrov et al. 2018; Kamrani et al. 2022; Sarraf et al. 2022). Hence, water deficit stress induces adaptive responses at low doses and causes adverse effects at high doses (Chaves et al. 2002), responses that are driven by foliar elements (Marschner 2012). For example, K participates in the detoxification of reactive oxygen species and exerts controls over stomata, photosynthesis, and water relations (Aliniaeifard et al. 2020). Trees, including larches, have diverse mechanisms that enable them to cope with drought. For instance, they can acclimate to annual variations in soil moisture level by adjusting the vertical distribution of their roots (Takenaka et al. 2016). Japanese larch copes with drought (up to some extent) by developing fine roots to promote water uptake and by increasing the root to shoot ratio (Takenaka et al. 2016). However, the opposite may also hold true for Japanese larch trees that are adapted to low-moisture environments and then exposed to considerably wet conditions (Takenaka et al. 2016). Adjustments to changes in moisture levels via alterations in root structure and functioning, including fine roots and root to shoot ratio, implies concomitant changes in nutrient dynamics, especially in carbon and nitrogen (Chaves et al. 2002; Lisar et al. 2012), and in the recruitment of root symbionts (e.g., ectomycorrhizae) that aid plant access to water and nutrients.
Similar to drought, fertilizers can increase root length and surface area and aboveground biomass and alter the ratio of root mass to total plant mass of Japanese larch in a dose-dependent manner (Qu et al. 2003). That is, a low-dose fertilization (e.g., 10 mg N plant–1) increases the root mass fraction, whereas a higher dose (e.g., 40 mg N plant–1) decreases the fraction of root mass to total plant mass (Qu et al. 2003). Hence, soil fertilization is expected to mediate the effect of environmental stress such as drought on Japanese larch. Importantly, the effects of fertilization on the roots suggest that symbioses between plants (fine roots) and ectomycorrhizae may be altered.
Fertilization regimes and microbes, such as plant growth-promoting rhizobacteria, arbuscular mycorrhizae and ectomycorrhizae, can also mitigate stress of water deficits in plants (Svenson et al. 1991; Alvarez et al. 2009; Sun et al. 2017; Bernardo et al. 2019; Aryal et al. 2020; Ahluwalia et al. 2021; Pons and Müller 2022). Such microorganisms and interactions can enhance the antioxidant system and phytohormones, improve nutrients and water uptake and relations, metabolism, root and shoot growth and vigor, increase photosynthetic pigments and rate, and decrease mortality in plants subjected to drought (Svenson et al. 1991; Alvarez et al. 2009; Sun et al. 2017; Bernardo et al. 2019; Aryal et al. 2020; Ahluwalia et al. 2021). Proper fertilization regimes can promote similar beneficial effects including an increase in photosynthetic pigments and photosynthetic rate, enhanced antioxidant system, improved water relations, and increased uptake and content of nutrients in plants under drought (Tariq et al. 2018; Shehab and Guo 2021). However, numerous factors such as the intensity and type of nutrient treatment and the age of the mycorrhizal symbiosis can influence the outcomes of experimental manipulations to alleviate water deficit stress (Sun et al. 2017; Shehab and Guo 2021; Pons and Müller 2022). Hence, the results differ among studies, and microbial inoculation and fertilization do not always alleviate drought effects (Emery et al. 2020; Kitao et al. 2022; Pons and Müller 2022).
In this study, we aimed to evaluate the effects of low and high soil fertilization and/or low and high soil water availability on container-grown seedlings of Japanese larch after inoculation with an ectomycorrhizal source (or blank inoculation). Container-grown seedlings have attracted interest for reforestation practices in Japan because they have greater survival rates compared to bareroot seedlings (Masaki et al. 2017; Agathokleous et al. 2020; Kitao et al. 2022). Hence, we designed this study within the context of forestation practice to enhance the performance of Japanese larch seedlings under water challenges.
Materials and methods
Experimental facility
The experiment was conducted in an automated glasshouse at the Hokkaido Research Center, Forestry and Forest Products Research Institute, Sapporo, Japan in 2018. The top and side windows were always open in the summer to prevent very high temperatures. The average daily mean, maximum, and minimum air temperature in the glasshouse during the specific duration of the experiment was 18.2 (SD: ± 5.2), 26.8 (SD: ± 6.4), and 12.8 (SD: ± 4.9) °C, respectively. The respective values were 16.1 (SD: ± 5.0), 22.6 (SD: ± 5.6), and 11.2 (SD: ± 5.2) °C in the outdoor air. The mean relative humidity was 71.3 % (SD: ± 10.7 %) in the glasshouse and 80.1 % (SD: ± 9.5%) outside the glasshouse.
Experimental materials, treatments, and design
Japanese larch (L. kaempferi) seeds were obtained from naturally growing mother trees on the Hokkaido and stored at 0 °C in a walk-in refrigerator (PR-22CC-1.5, Hoshizaki Corporation, Toyoake, Japan). Early in the spring of the experimental season, seeds were moved to a lab and disinfested. On 10 May, randomly selected seeds were sown in 0.2-L containers (5.5 cm diameter, 13 cm high) that are used in the local forestry industry because of the increased survival rates relative to bareroot seedlings (Masaki et al. 2017). Four seeds were sown in top-cocopeat (Top, Osaka, Japan; see Supplementary Materials for components) in each pot. The substrate was then kept moist with tap water until transplantation as explained later. The containers were placed on bases, each of which had 35 holes especially designed for inserting the containers, according to local reforestation practice. Ten seedlings were inserted in randomly selected holes in each base and their position rotated biweekly; the design of the entire experiment was fully randomized. The plastic bases were placed on different tables, at a height of 1 m in rows 2 m apart. The position of the bases (and containers) was rotated within and across tables on a weekly basis. There were 80 plants in total, and they were cultivated in ambient conditions.
One month after sowing (5 June), 40 seedlings were randomly allocated into one of two fertilization treatments, treated with slow-release Osmocote Exact Standard 8-9M fertilizer (15-9-11 + 2MgO + TE, Hyponex Japan Corp. Ltd, Osaka, Japan) at a dose of 0.5 g (low-dose fertilization [LF]) or 2 g (high-dose fertilization [HF]). Seven days later, the seedlings were thinned to one per container to keep the most vigorous for further cultivation and experimentation. On 22 June, an irrigation system was installed. Each container was connected to one tube which in turn was attached to the bottom of an inversely positioned syringe (without the inner plastic mechanism for creating pressure) that was placed 1 m above the tables. At the edge of the tube that was connected to the pot, a 200-μL pipette tip (first 2–3 mm cut at a diagonal and removed) was attached to ensure a slower flow of irrigation water into each container. From this point onward, plants were irrigated twice a week (Monday and Friday) with 70 mL water in morning and in the evening until transplantation.
One month after the fertilization treatment (10 July), ECM treatments were conducted based on a previous experience (Agathokleous et al. 2020). Roots of 6-year-old L. kaempferi saplings in the Sapporo Experimental Forest of Hokkaido University (43°04′ N, 141°20′ E) were dug out. Based on perfectly formed ectomycorrhizal tips and hyphal network according to previous experience of visual observations and DNA analyses of ECM in this species (Wang et al. 2015; Agathokleous et al. 2020), a considerable portion of roots that had formed ECM was removed and used immediately as inoculum. Five liters of tap water were added to each of two 20-L containers, then the ECM inoculum was added to one container and the mixture agitated for 15 min. The ECM inoculum was then removed and placed in the other container (and vice versa) and agitated for 15 min. The solution in one container was sterilized by boiling for 40 min, then 5 L of cold tap water were added to each container. Half of the experimental seedlings from each fertilization treatment were randomly allocated into one of the two ECM treatments: half were irrigated with 60 mL of non-sterilized solution (ECM) and the rest were irrigated with 60 mL of sterilized solution (non-ECM). In a previous experiment, plants developed damping off approximately 1 month after inoculation (Agathokleous et al. 2020). However, in the current experiment, no damping off occurred.
Approximately 1.5 month after the ECM inoculation (24 September), plants were transplanted into 12 L pots and irrigated with 500 mL of tap water. For each experimental condition, 3–6 seedlings were randomly selected and evaluated for physiological and growth status (described in “Pre-drought evaluation of plant-level performance” section). The remaining plants were moved into an adjacent, interconnected glasshouse with identical conditions for further experimentation. Starting from 2 October, at 7-day intervals until the end of the experiment, half of the seedlings in each irrigation treatment were given 500 mL of tap water (well-watered, no-drought; WW), while the other half were given 50 mL of tap water (drought; DR). The DR treatment lasted 1.5 month, which is long enough in northern Japan, where there is periodic precipitation in normal years. For instance, there were unusually high temperatures and a prolonged drought during the summer 2021, with the longest drought lasting 1.5 months (Japan Meteorological Agency; https://www.jma.go.jp/jma/index.html). The treatments are outlined in Table 1.
Data collection
Pre-drought evaluation of plant-level performance
Seedlings were assessed for effects of the fertilization and ECM treatments on 24 September to establish the baseline condition before the irrigation treatment began. The sampled seedlings received 140 mL of water the day before measurements (23 September). For water potential measurements, 3–6 randomly selected seedlings for each treatment were transferred to a dark room on the evening of 23 September. Before dawn the next day, one randomly selected branch was removed from each seedling to measure predawn water potential using a Model 1505D-EXP Pressure Chamber Instrument (PMS Instrument, Albany, OR, USA). All buds on each plant were also counted. Two crosswise measurements of the stem basal diameter, using a digital vernier caliper with a two-digit accuracy, were taken and averaged. Stem height was measured using a measuring tape with a 1-mm grading, as the distance from the soil line at the base of the stem to the stem apex. The crown width of each seedling was recorded as the average of two crosswise measurements of the two farthest points of the crown as observed from above, using a measuring tape with a 1-mm grading. Seedlings were harvested as an entire unit along with their root system, which was gently extracted from the soil and washed with tap water to remove foreign particles. The length of the longest root was measured with a measuring tape (1-mm grading) as the distance from top to bottom. The seedlings were separated into different compartments and air-dried at 75 °C to constant mass. Root, foliage, and stem + branches dry matters were weighed with a digital balance with an accuracy of four digits. Aboveground (shoot) biomass was calculated as the sum of foliage mass and stem + branches mass. Total plant biomass was calculated as the sum of shoot biomass and root biomass. The ratio of the root length to root dry mass was calculated as a potential index to indicate whether effects on root dry mass were due to increased root length. The root mass to shoot mass ratio (R/S) was also calculated.
Gas exchange was analyzed with the LI-6400 Portable Photosynthesis System (LI-COR, Lincoln, NE, USA). Photosynthetic rate (A390), stomatal conductance (Gs390), and transpiration rate (E390) were measured with a leaf temperature of 25 °C at a CO2 concentration of 390 μmol mol−1, a relative air humidity of 60 ± 5%, and a light intensity of 1500 μmol m−2 s−1. Following recording of data, the light intensity was decreased to 100 μmol m−2 s−1, and A390 and Gs390 were measured again after 15 min. The response to light was calculated as the percentage difference of the values recorded at 100 μmol m−2 s−1 relative to the values recorded at 1500 μmol m−2 s−1. Measurements were taken in the morning using sunlit mature needles, selected randomly. The needles were scanned (Canon LIDE 40, Tokyo), and their projected area was estimated using the software LIA-32 (Yamamoto 2003). Measurements were often taken from more than one set of needles per plant, and the average was calculated as a more representative estimate for statistical analysis. Seedlings from different experimental conditions were measured in rotation to account for any temporal effect of methodological error. Gas exchange was measured on work days during 5–12 September (1st time; see “Post-drought evaluation of plant-level performance” section for next times). Gas exchange was measured for 8 or 9 randomly selected seedlings per experimental condition after re-irrigation of all seedlings with 140 mL water.
Post-drought evaluation of plant-level performance
Final assessment of the impacts of fertilization, ECM, and water treatments was carried out prior approximately 1.5 month after water treatments (15 November). Therefore, this assessment is also considered baseline condition for the irrigation treatment. Six to nine seedlings per experimental condition were evaluated for stem basal diameter, stem height, crown spread, and number of buds as described for the pre-drought evaluation (see “Pre-drought evaluation of plant-level performance” section). The plants were harvested, separated into different organs, which were then weighed as described for the pre-drought evaluation (see “Pre-drought evaluation of plant-level performance” section). Any leaves shed from each plant remained intact on the soil surface in each pot and were collected, dried, and weighed. Aboveground biomass, total plant biomass, and R/S ratio were calculated as in “Pre-drought evaluation of plant-level performance” section. For this evaluation, foliage, aboveground, and total plant biomass also includes the shed needles. To understand whether the treatments affected the senescence rate, the ratio of the mass of shed needles to the mass of foliage (all needles) was also calculated.
The morphology of the fresh roots was examined using a Leica MS5 stereomicroscope (Leica Microsystems) with five-step magnification changer, trinocular tube mounted digital camera, and a connected screen.
Water potential was measured in randomly selected branches in all seedlings right before harvest. Since most of the seedling consists of woody organs, water potential of the branches was measured to reflect plant-level water status. Each seedling received 500 mL of water the day before water potential was measured as described in “Pre-drought evaluation of plant-level performance” section.
Gas exchange was analyzed using the methodology in “Pre-drought evaluation of plant-level performance” section; however, light response was not evaluated post-drought. Gas exchange was measured on work days during 10–12 October (2nd time), 31 October–2 November (3rd time), and 6–8 November (4th time). Gas exchange was measured for 3–7 randomly selected seedlings per experimental condition in October–November (after irrigation treatments). Measurements at times 2 (1 week after drought) and 4 (5 weeks after drought) were done after re-irrigation of all seedlings with 500 mL of water, whereas measurements at time 3 (4 weeks after drought) were done 1 week after re-irrigation.
After drying, the foliage of each plant or for two plants of the same experimental conditions, randomly selected, was pooled and ground into a fine powder, which was then digested with HNO3 and H2O2. PP tubes (50 mL, DigiTUBES, SCP Science) and a thermal unit (105 °C; DigiPREP, SCP Science) were used. The residues from the digestion were then diluted with 2% (v/v) HNO3 and filtered through a Teflon membrane filter (0.45 µm; DigiFILTER, SCP Science). P, K, and Mg concentrations were assessed with an inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7700a, Agilent Technologies, Santa Clara, CA, USA).
Data analysis
The a priori level of statistical significance was α = 0.05. Due to significant (P < 0.05) deviation of the data of several variables from the standard Gaussian distribution, all data sets were subjected to a power Box-Cox transformation (Box and Cox 1964; Agathokleous et al. 2016).
The data for the pre-drought plant-level assessment and gas exchange measured in September (time 0) were analyzed using a σ-restricted generalized linear model (GLM) with type VI sum of squares, with fertilizer and ECM as fixed factors, each with two levels. The data for the post-drought final plant-level assessment and foliage elements were analyzed using a σ-restricted GLM with type VI sum of squares, with fertilizer, ECM, and irrigation as fixed factors, each with two levels. Gas exchange data for October–November (times 1, 2, and 3) were analyzed using a σ-restricted GLM with type VI sum of squares, with fertilizer, ECM, and irrigation as fixed factors, each with two levels, and irrigation as repeated (within effects) factor with three levels. Gas exchange data for September were not included into the repeated measures analysis because different seedlings were measured randomly each time, and if only the seedlings with common measurements across all times were used, the number of statistical units would be insufficient for analysis. The stem height and aboveground biomass of LF- and HF-treated seedlings of the present experiment (all other conditions pooled) were compared with the respective stem height and aboveground biomass of LF- and HF-treated seedlings of a previous experiment (all other conditions pooled; Agathokleous et al. 2020) using an independent-sample t-test. The data supporting the latter analysis are presented in the respective figures in this and the previous study (Agathokleous et al. 2020); thus, only the statistical results are mentioned when referring to these results (see Supplementary Materials).
For any significant interactions based on GLM analyses, multiple comparisons between experimental conditions (groups) were performed using a t-test. To account for trade-offs between type I and type II errors, considering also the influence of α correction, only the most biologically meaningful comparisons were selected a priori for analysis. For fertilizer × ECM interaction, the comparisons were (1) non-ECM × HF versus non-ECM × LF, (2) ECM × HF versus ECM × LF, (3) LF × non-ECM versus LF × ECM, and (4) HF × non-ECM versus HF × ECM. Regarding ECM × irrigation the pairs of comparison were (1) ECM × DR versus ECM × WW, (2) non-ECM × DR versus non-ECM × WW, (3) DR × ECM versus DR × non-ECM, and (4) WW × ECM versus WW × non-ECM. The pairs of comparison for the fertilizer × irrigation interaction were (1) LF × DR versus LF × WW, (2) HF × DR versus HF × WW, (3) DR × HF versus DR × LF, and (4) WW × HF versus WW × LF. For the interaction fertilizer × ECM × irrigation, the comparisons were same as for ECM × irrigation, but for each fertilization treatment separately, giving a total of eight comparisons (see Table S1). Nine comparisons were tested for the time × irrigation interaction, namely between the two irrigation treatments within each time and between times within an irrigation treatment (see Table S2 for the exact pairs).
To evaluate the magnitude of treatment effect, effect size was calculated for comparisons between groups for which there was some evidence for a statistically significant difference. Specifically, Cohen’s δ (Cohen 1988) was calculated as described previously (Agathokleous et al. 2016). Absolute values of δ in the arbitrary ranges of < 0.2, ≥ 0.2 < 0.5, ≥ 0.5 < 0.8, and > 0.8 indicate a neutral, small, moderate, and large effect, respectively, whereas values in the ranges of 0.25–0.5 and > 0.5 indicate educational and practical significance, respectively (Tallmadge 1977; Wolf 1986; Cohen 1988; Agathokleous et al. 2016).
Results are presented as means ± standard error (SE), whereas the 95% confidence interval (CI) of the effect size is also reported; for CIs whose upper bound is negative, “to” is used (thus, only “-” before the value of the upper bound indicates a positive value). The number of replicates for each experimental condition per trait is presented in the respective diagrams.
The data were processed and analyzed statistically with the software Excel 2010 (Microsoft, Redmond, WA, USA) and Statistica v.10 (StatSoft, Tulsa, OK, USA) and a freely available effect size estimation application (Agathokleous and Saitanis 2020).
Results
Pre-drought assessment of plant performance
Approximately 2.5 months after fertilization, 1.5 month after inoculation, and right before transplantation and irrigation treatments, there was no evidence that water potential was influenced by the experimental treatments (Supplementary Materials, Fig. S1).
For plant growth traits, there was no significant main effect of fertilizer on root length (Fig. 1A). However, relative to the effect of LF, HF had a large negative effect on the root length to root biomass ratio (δ = − 1.94, CI − 19.83 to 31.03; Fig. 1B) and a large positive effect on stem height (δ = 2.47, CI − 0.55 to 3.60; Fig. 1C), crown width (δ = 2.39, CI 0.07 to 3.30; Fig. 1D), stem diameter (δ = 3.01, CI 2.53 to 3.30; Fig. 1E), and number of buds per plant (δ = 2.04, CI − 9.18 to 6.42; Fig. 1F). There was weak evidence for a moderate negative effect of ECM on root length (δ = − 0.73, CI − 2.82 to 0.39; Fig. 1A). ECM had a moderate positive effect on the root length to root biomass ratio (δ = 0.59, CI − 40.82 to 32.78; Fig. 1B) and a moderate negative effect on stem height (δ = − 0.67, CI − 3.73 to 3.16; Fig. 1C), crown width (δ = − 0.86, CI − 3.02 to 1.95; Fig. 1D), and number of buds per plant (δ = − 0.71, CI − 10.38 to 12.01; Fig. 1F). There was no evidence for a significant fertilizer × ECM interaction for any of the growth traits (Fig. 1).
Means ± SE of plant growth traits. Japanese larch seedlings were sampled approximately 1.5 months after inoculation with ectomycorrhizae (ECM) and 2.5 months after fertilization (pre-drought assessment). LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Data were analyzed with a generalized linear model (GLM) at α = 0.05. n indicates the sample size for each experimental condition
For plant biomass production traits, compared to LF, HF led to a large increase in root mass (δ = 1.77, CI 1.60 to 1.83; Fig. 2A), foliage mass (δ = 2.22, CI 1.87 to 2.27; Fig. 2B), stem + branches mass (δ = 2.36, CI 2.24 to 2.38; Fig. 2C), aboveground biomass (δ = 2.33, CI 1.88 to 2.40; Fig. 2D), and total plant biomass (δ = 2.25, CI 1.65 to 2.38; Fig. 2E). Conversely, HF led to a large decrease in R/S ratio (δ = − 1.56, CI − 1.64 to 1.38; Fig. 2F). ECM treatment resulted in a large decrease in root mass (δ = − 0.94, CI − 1.03 to − 0.76; Fig. 2A), foliage mass (δ = − 0.82, CI − 1.00 to − 0.41; Fig. 2B), stem + branches mass (δ = 0.81, CI − 0.88 to − 0.67; Fig. 2C), aboveground biomass (δ = − 0.83, CI − 1.07 to − 0.28; Fig. 2D), and total plant biomass (δ = − 0.88, CI − 1.19 to − 0.16; Fig. 2E). No evidence was observed for ECM affecting R/S ratio (Fig. 2F). Evidence for a significant fertilizer × ECM interaction was found for foliage mass (Fig. 2B), aboveground biomass (Fig. 2D), and total plant biomass (Fig. 2E); for all three traits, ECM treatment largely decreased the positive effect of HF (Fig. 2; Supplementary Materials, Table S3).
Means ± SE of plant biomass production traits for Japanese larch seedlings approximately 1.5 months after inoculation with ectomycorrhizae (ECM) and 2.5 months after fertilization (pre-drought assessment). LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Different lowercase letters above error bars indicate a significant difference between LF and HF within non-ECM or within ECM. Different uppercase letters above error bars indicate significant difference between non-ECM and ECM within LF or within HF. Data were analyzed with a generalized linear model (GLM), followed by t tests at α = 0.05. n = sample size for each experimental condition
For gas exchange, there was no evidence for significant differences between experimental conditions before the drought treatment, although the distribution of Gs390 values of LF × ECM plants shifted well below the distributions of the values of the other three experimental conditions, indicating a trend toward lower values (Fig. 3). There was also no evidence that the treatments significantly altered response to light (Supplementary Materials, Fig. S2).
Means ± SE of plant gas exchange traits for Japanese larch seedlings approximately 1.5 months after inoculation with ectomycorrhizae (ECM) and 2.5 months after fertilization (pre-drought assessment). A390, net photosynthetic rate; Gs390, stomatal conductance; E390, transpiration rate. LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Data were analyzed with a generalized linear model (GLM) at α = 0.05. n = sample size for each experimental condition
Post-drought assessment of plant performance (after water treatments)
The final impacts of all experimental treatments on plants were evaluated approximately 5, 4, and 1.5 months after fertilization, ECM, and irrigation treatments, respectively.
Growth and biomass
Similar to results of the pre-drought assessment (“Pre-drought assessment of plant performance” section), HF had various positive effects on plants, compared to LF (regardless of the ECM and irrigation treatments). It caused a large increase in stem diameter (δ = 3.02, CI 2.69 to 3.24; Supplementary Materials, Fig. S3), stem height (δ = 2.97, CI 0.98 to 3.59; Fig. S4), crown width (δ = 3.08, CI 1.60 to 3.64; Fig. S5), number of buds per plant (δ = 3.06, CI − 4.98 to 5.83; Fig. S6), foliage mass (δ = 2.97, CI 2.80 to 3.01; Fig. S7), stem + branches mass (δ = 3.18, CI 3.04 to 3.21; Fig. S8), aboveground biomass (δ = 3.17, CI 2.88 to 3.24; Fig. S9), root mass (δ = 2.11, CI 1.82 to 2.20; Fig. S10), and total plant biomass (δ = 2.80, CI 2.26 to 2.96; Fig. S11). Conversely, HF led to a large decrease in R/S ratio (δ = − 1.13, CI − 1.22 to − 0.83; Fig. S12).
ECM treatment negatively affected plants, compared to non-ECM (regardless of fertilization and irrigation treatment), similar to the results of the pre-drought assessment (“Pre-drought assessment of plant performance” section). ECM treatment had a moderate negative effect on stem diameter (δ = − 0.60, CI − 1.03 to − 0.10; Fig. S3), stem height (δ = − 0.61, CI − 2.57 to 1.95; Fig. S4), crown width (δ = − 0.53, CI − 1.94 to 1.59; Fig. S5), stem + branches mass (δ = − 0.53, CI − 0.65 to − 0.34; Fig. S8), and total plant biomass (δ = − 0.50, CI − 1.00 to 0.18; Fig. S11). It also had a small negative effect on foliage mass (δ = − 0.41, CI − 0.58 to − 0.20; Fig. S7), aboveground biomass (δ = − 0.47, CI − 0.76 to − 0.08; Fig. S9), and root mass (δ = − 0.48, CI − 0.70 to − 0.16; Fig. S10).
Irrigation main effect was significant for the ratio of shed needles mass to all needles mass (Fig. 4A), with DR largely increasing the ratio relative to WW (δ = 0.92, CI 0.86 to 1.00).
Means ± SE of the ratio of shed needles mass to all needles mass (A) and branch water potential (B) for Japanese larch seedlings approximately 5, 4, and 1.5 months after low or high fertilization, inoculation with ectomycorrhizae (ECM), and irrigation treatments (final [post-drought] assessment). WW, well-watered (500 mL weekly) plants; DR, drought-treated plants (50 mL weekly); LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Factors: F, fertilizer; E, ECM; I (in non-italics) irrigation. Data were analyzed with a generalized linear model (GLM) at α = 0.05. n = sample size for each experimental condition
The only significant interaction was ECM × irrigation for the ratio of shed needles mass to all needles mass (Fig. 4A). The only significant difference in the tested pairs of comparisons was between ECM × DR and. ECM × WW (Table S4), where DR resulted in a large increase in the ratio, compared to WW, in ECM-treated plants (δ = 1.32, CI 1.23 to 1.45; Fig. 4A).
Water potential
Regarding branch water potential, only irrigation and the interaction of fertilization × ECM × irrigation were significant factors (Fig. 4B). DR largely decreased the water potential, compared to WW (δ = − 2.53, CI − 2.57 to − 2.50). The only statistically significant comparisons were those of (1) LF × non-ECM × WW versus LF × non-ECM × DR, (2) LF × ECM × WW versus LF × ECM × DR, (3) LF × non-ECM × DR versus LF × ECM × DR, (4) HF × non-ECM × WW versus HF × non-ECM × DR, and (5) HF × ECM × WW versus HF × ECM × DR (Table S1). The differences between these pairs were large (Table S1).
Root morphology
Roots of HF × non-ECM × WW seedlings had many root tips that formed ECM, and also some amount of root hairs, which directly absorb water and nutrients, thus preventing enhanced ECM symbioses due to self-sufficient water support (Fig. S13). Roots of HF × non-ECM × DR seedling had many root hairs, but less ECM (Fig. S14). Roots of HF × ECM × DR seedling had abundant root hairs, few ECM, and even the root tips with ECM had root hairs, indicating that the ECM had not completely formed (Fig. S15). Roots of LF × ECM × DR seedling had some ECM and very few root hairs (Fig. S16). Roots of LF × non-ECM × DR seedling had abundant ECM (the most observed) and almost no root hairs (Fig. S17).
Gas exchange
For post-irrigation gas exchange, fertilization was a significant main factor (Fig. 5, Table S5). Specifically, HF resulted in a small decrease in Gs390 (δ = − 0.40, CI − 0.41 to − 0.38; Fig. 5B) and E390 (δ = − 0.41, CI − 0.58 to − 0.20; Fig. 5C), while its effect on A390 was insignificant (δ = − 0.10, CI − 1.71 to 1.78; Fig. 5A), relatively to LF. Irrigation was also a significant factor (Fig. 5, Table S5), with DR largely suppressing A390 (δ = − 2.20, CI − 2.32 to − 2.07), Gs390 (δ = − 2.15, CI − 2.15 to − 2.14), and E390 (δ = − 2.20, CI − 2.32 to − 2.07), compared to WW. ECM was a nonsignificant factor (Fig. 5, Table S5). Among all the interactions, only time × irrigation interaction was significant and only for Gs390 and E390 (Fig. 5, Tables S2, S5).
Means ± SE of plant gas exchange traits for Japanese larch seedlings before (time 0) and after (times 1, 2, 3) irrigation treatments began (see “Data collection” section for details about the dates). A390, net photosynthetic rate; Gs390, stomatal conductance; E390, transpiration rate; ECM, inoculation with ectomycorrhizae; WW, well-watered (500 mL weekly); DR, drought (50 mL weekly); LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Data were analyzed with a generalized linear model (GLM) at α = 0.05, and the results are provided in Supplementary Materials (Tables S2, S5). Time 0: before drought treatment; data are baseline measurements and are the average of all the experimental conditions at that time (see data in Fig. 3); wk(s), week; d, day(s)
Foliar elemental contents
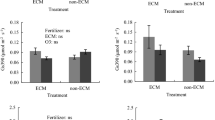
HF had a large positive effect on P (δ = 0.97, CI 0.84 to 1.12; Fig. 6A) and K (δ = 0.93, CI − 0.25 to 1.86; Fig. 6B) and a moderate to large positive effect on Mg (δ = 0.78, CI 0.53 to 0.99; Fig. 6C), compared to LF. Irrigation was not a significant factor for P. DR caused a large increase in K (δ = 0.88, CI − 0.10 to 1.60; Fig. 6B) and a moderate to large decrease in Mg (δ = − 0.79, CI − 1.04 to − 0.56; Fig. 6C) relative to WW.
Means ± SE of phosphorus (A), potassium (B), and magnesium (C) in foliage of. Japanese larch seedlings approximately 5 months after fertilization, 4 months after ECM inoculation (ECM), and 1.5 months after irrigation (final assessment). WW, well-watered (500 mL weekly); DR, drought (50 mL weekly); LF, 0.5 g (low-dose fertilization); HF, 2 g (high-dose fertilization) 15-9-11 + 2MgO + TE fertilizer. Factors: F, fertilizer; E, ECM inoculation; I, irrigation. Data were analyzed with a generalized linear model (GLM) at α = 0.05. n = sample size for each experimental condition
The interaction fertilizer × ECM was significant only for K (Fig. 6B). Among the four pairs of comparisons tested (see “Data analysis” section), only the comparison between non-ECM × HF and non-ECM × LF was significant. Specifically, non-ECM plants grown with HF had a large increase in K content (δ = 1.53, CI − 0.19 to 2.40; Fig. 6B) relative to non-ECM plants grown in LF.
Fertilizer × irrigation interaction was significant only for P (Fig. 6A) and K (Fig. 6B). Within the interaction, the only non-significant pair of comparison was that of WW × HF versus WW × LF for P. When grown in LF, foliage of DR-subjected plants was largely impoverished in P relative to WW plants (δ = − 1.53, CI − 1.52 to − 1.38; Fig. 6A). However, when grown in HF, foliage of DR-exposed plants was moderately enriched in P relative to WW plants (δ = 0.72, CI 0.53 to 0.87). Under DR stress, HF-treated plants had largely enhanced P content compared to LF-treated plants (δ = 2.07, CI 1.88 to 2.22); a difference that was not observed in WW (Fig. 6A). Regarding K, the pairs LF × DR versus LF × WW and WW × HF versus WW × LF were not significant, but the pairs HF × DR versus HF × WW and DR × HF versus DR × LF were significant. Specifically, within HF-treated plants, DR led to a large increase in K relative to WW (δ = 2.04, CI 0.71 to 2.94; Fig. 6B). Within DR, HF also largely enhanced K content relative to LF (δ = 1.88, CI 0.55 to 3.38; Fig. 6B).
ECM × irrigation interaction was significant only for P (Fig. 6A). No significant difference was observed for the pairs non-ECM × DR versus non-ECM × WW and DR × ECM versus DR × non-ECM. However, the comparisons between ECM × DR and ECM × WW and between WW × ECM and WW × non-ECM were significant. As to the former pair, ECM-treated plants had a moderately lower P content when exposed to DR, compared to WW (δ = − 0.76, CI − 1.00 to − 0.62; Fig. 6A). Regarding the latter pair, within WW, ECM-treated plants were largely enriched in P content, compared to non-ECM plants (δ = 1.17, CI 1.03 to 1.32; Fig. 6A).
ECM and the interaction fertilizer × ECM × irrigation were not significant main factors for P, K, and Mg.
Discussion
The single and combined treatments of container-grown Japanese larch seedlings with different fertilization rates, ECM inoculum, and irrigation revealed various micro- and macro-level effects on plants, from leaf-level physiology to individual organism. However, due to the limited evidence for significant effects of multifactorial interactions, the results are discussed for individual factors. The three factors are discussed in sequence of experimental manipulation: fertilization, ECM, and irrigation. The few significant interactions are mainly discussed in the section on the respective factor (interaction term) that succeeded the other factors in the interaction. All the significant effects or differences between experimental conditions that are discussed hereafter are educationally significant and most of practical importance.
Fertilization
Fertilization of seedlings is vital for their vigor and success after they are transplanted. In this study, HF caused moderate to large increases in P, K, and Mg, as expected based on the composition of the fertilizer. This practically-significant enrichment of major elements by HF showcases that plants accessed and transported significant amounts of the applied fertilizer to the needles. These results confirm the validity of fertilization treatment and can explain the enhanced growth and productivity by these macronutrients that drive plant growth and development (Guo et al. 2015, 2016; Malhotra et al. 2018; Hauer-Jákli and Tränkner 2019; Johnson et al. 2022).
A number of studies show that proper fertilization over several weeks or multiple years benefits Japanese larch plantations (Kochenderfer et al. 1995; Yi et al. 2002; Qu et al. 2003). However, the effect of fertilization on container-grown seedlings within the context of this recent modern forestry practice remains underexplored (Agathokleous et al. 2020). Here, the large enhancement of stem diameter and height, crown width, number of buds per plant, root mass, foliage mass, stem + branches mass, aboveground biomass, and total plant biomass by HF, relative to LF, illustrates the practical significance of this fertilization regime for forestry practice to produce superior container-grown seedlings. A previous study revealed that HF enhanced the growth and productivity of Japanese larch container-grown seedlings (Agathokleous et al. 2020). However, the aims of that study differed, and a large part of that study was done in an open field where the plants were rainfed. In this present study, seedlings were grown in a glasshouse, according to the current forestry practices and subjected to strict irrigation schemes, except for the seedlings of DR treatment. This improved cultivation practice within a forestry framework resulted in seedlings grown with HF being largely superior to those in the study by Agathokleous et al. (2020) (see Supplementary Materials). Thus, the present protocol offers a practically significant benefit for production of elite seedlings of Japanese larch for forestation.
As a result of HF treatment, the R/S ratio was largely lower relative to the ratio in LF-treated plants and is attributed to the greater enhancement of shoot relative to root. The R/S ratio is an important index of plant health, and its use is warranted for producing container-grown seedlings for forestry (Agathokleous et al. 2019; Harayama et al. 2021). Seedlings with relatively larger shoots are more competitive for aboveground resources (e.g., sunlight) and better survival and establishment after transplanting, assuming that the belowground resources (e.g., water, nutrients) are sufficient. However, if seedlings are to be planted in environments with limited belowground resources, a relatively greater root system may be beneficial (Harayama et al. 2021). In this study, when evaluated before drought treatments, HF did not significantly change the root length but largely decreased the root length to root biomass ratio, suggesting that HF-treated plants had a denser root system characterized by greater fractal dimensions. This difference of the root system of HF-treated plants was so large that would be visible to the naked eye of even the most careless observer by the end of the experiment (Supplementary Materials, Fig. S18). This indicates an increased capacity to directly access water and nutrients, and can explain why roots of HF-treated plants formed less complete ectomycorrhizal root tips.
There was no significant evidence for HF effect on gas exchange, response to light, and water potential before irrigation treatments begun or on water potential at the end of the experiment. However, HF caused a small decrease in Gs390 and E390 that was not of significant practical consequence based on data from the three measurements after the initiation of the irrigation treatments to the end of the experiment. Decreases in Gs and E after short-term fertilization were found in other studies using different tree species too, although the exact underlying physiological mechanisms remain unclear (Ward et al. 2015). A potential reason may be structural changes in leaves; for example, fewer and/or smaller stomata in relation to a greater leaf area index caused by fertilization, considering that Gs and E represent leaf area-based indexes (Paoletti and Grulke 2005). However, other reasons might be at play too, such as shifts in the conductivity and/or structure of the hydraulic system as well as altered root area, which was largely increased by HF in this experiment (Ewers et al. 2000; Ward et al. 2015). The enriched K content in the foliage suggests that the decreased Gs was not due to K limitation; K is important for plant growth (Johnson et al. 2022). Across various plant species, the critical Mg concentration (%) below which dry mass is negativly affected ranges from 0.1% to 0.2% (Hauer-Jákli and Tränkner 2019). In this experiment, the average was 0.22% for LF and 0.27% for HF, values that are greater than the overall range across species (Hauer-Jákli and Tränkner 2019).
ECM treatment
Contrary to our expectations, ECM treatment caused a series of negative effects on seedlings, without offering any benefits, although there was no evidence for significant effects of ECM treatment on gas exchange or water potential at any time. At the pre-drought assessment (before irrigation began), ECM treatment caused a practically significant stress in seedlings, as indicated by a moderate inhibition of stem height, crown width, number of buds per plant, plant biomasses, and root length. Concurrently, ECM increased the root length to root biomass ratio. These results suggest that ECM-treated plants had a sparser root system, likely characterized by smaller fractal dimensions. While there was no evidence for significant fertilizer × ECM interaction for the studied growth traits, ECM treatment largely counteracted the enhancement of foliage, aboveground, and total plant biomass of HF-treated seedlings. In agreement, ECM treatment appeared to counteract the enrichment of foliar K content by HF, which occurred at a large extent in non-ECM plants. Although the underlying biological mechanism of this effect remains unclear, HF-treated plants under stress after microbial inoculation might consume more K for detoxification and defense (Johnson et al. 2022). These observations suggest that ECM treatment produced more practically relevant negative effects in the presence of HF than in LF. Various negative effects of ECM treatment were still observed at the end of the experiment, many of which were practically important. These results indicate that seedlings suppressed by ECM earlier in the experiment could not recover the damage by the end of the growing season.
Controlled, species-specific mycorrhization can offer various benefits to trees and agronomical crops, such as enhanced CO2 assimilation, improved water relations, and increased root growth, although not always revealed when present (Svenson et al. 1991; Maltz et al. 2019; Ulrich et al. 2019; Hu and Chen 2020; Corsini et al. 2022; Pons and Müller 2022). Moreover, although such controlled mycorrhization may alleviate the effects of stressful conditions (Yin et al. 2017; Maltz et al. 2019), some studies show that initial enhancement of plant performance by mycorrhization can turn into negative effect during severe stress such as a water deficit (Ulrich et al. 2019). Interestingly, counter to our expectations, there was some evidence that non-ECM seedlings had even more ectomycorrhizal root tips and complete ectomycorrhizae than did ECM-treated seedlings, indicating that the inoculation protocol was unsuccessful. These observations suggest that, although ECM treatment negatively affected symbioses and plants overall, ectomycorrhization in the non-inoculated plants had a positive effect on plants compared to the inoculated plants with fewer root tips and more incomplete ectomycorrhizae.
The findings of significant negative effects of ECM treatment on seedlings agree with the findings in the previous experiment in which ECM-treated Japanese larch seedlings also lacked complete formation of mycorrhizae at the root tips (Agathokleous et al. 2020). Because seedlings developed damping off in the previous experiment, and DNA analyses revealed potentially pathogenic Fusarium sp. in ECM-treated plants, the previous protocol was likely unsuccessful because of contamination. However, the protocol was unsuccessful in the present experiment too, even though the seedlings were considerably vigorous, healthy, and had no disease symptoms. Why the roots of non-ECM inoculation had many ectomycorrhizae, and the roots of ECM-inoculated plants had fewer ECM is unknown. However, we hypothesize that the ECM inoculum directly stressed plants early after treatment, which in turn indirectly suppressed the formation of complete ECM due to limited resources available to ECM. We also speculate that, regardless of the vigor of ECM-inoculated seedlings, microbes other than ECM in the inoculum might outcompete ECM. These findings are important because they suggest that general inoculum from older, large trees in the field may be unsuitable for establishing juvenile seedlings. They further suggest that the rhizosphere microbial communities of generating seedlings might differ from those of large, old trees, and the microbiome of the old trees might cause an imbalance in the microbiome of the seedlings. For example, the microbiome in the rhizosphere of the older trees might keep the non-ECM species in a nonpathogenic state, whereas rhizosphere microbiome of the seedlings cannot. These possibilities have important consequences for the forestry practice and need further testing. Nevertheless, these findings indicate the important role of ectomycorrhizae in producing healthier and more vigorous plants as reflected by the superior seedlings of non-ECM treatment with greater growth and biomass and more root tips and complete ectomycorrhizae compared to the inferior ECM-treated seedlings.
Irrigation
Plants are sensitive to drought and may be more sensitive to water deficit stress followed by re-irrigation or a cycled change in water status, which is expected to be a more frequent phenomenon under climate change (Xu et al. 2009, 2010; Turcsán et al. 2016). In this study, compared to the WW treatment, DR treatment greatly decreased A390, Gs390, E390, and water potential and decreased the ratio of shed needles mass to all needles mass. These results indicate that CO2 assimilation decreased due to stomatal closure and needle senescence induced by DR-induced oxidative stress. Moreover, these negative effects of DR treatment were driven by a large decrease in foliar Mg content, a major nutrient involved in stress signaling, growth, and development (Guo et al. 2015, 2016; Hauer-Jákli and Tränkner 2019). Reduction in Mg leads to a lower A, as found in the present study and others (Guo et al. 2015, 2016; Hauer-Jákli and Tränkner 2019). An analysis of published research indicated that the critical Mg concentration (%) below which negative effects on net CO2 assimilation occur ranges from 0.05% (in Pinus radiata) to 0.72% (in Helianthus annuus) (Hauer-Jákli and Tränkner 2019). In our experiment, DR treatment decreased Mg from 0.28% in WW to 0.23%. While the numerical difference might seem small, statistically it was large and in line with the inhibition of A390.
The large increase in K due to DR further suggests an important role of K in water deficit stress. K participates in regulating stomatal opening, photosynthesis, osmotic adjustment, and water balance; detoxificating reactive chemical species; and protecting against drought and other abiotic stressors (Hu and Schmidhalter 2005; Johnson et al. 2022). The activation of over 60 enzymes depends on K (Johnson et al. 2022). The large increase in K content by DR may indicate a mechanism to reduce reactive chemical species under water deficit stress and is in line with emerging evidence that augmented K+ nutritional status can decrease reactive oxygen species and promote tolerance to abiotic stress (Pandey and Mahiwal 2020). Under DR, accumulating K+ might be more critical relative to producing organic solutes at the initial phase of adjustment, due to higher energetic efficiency of osmotic adjustment via the uptake of ions such as K+ (Hsiao 1973; Hu and Schmidhalter 2005). A swift discharge of K+ from the guard cells to the apoplast is succeeded by stomatal closure, and K impoverishment may impede keeping stomata open (Wang et al. 2013). Some experiments indicated that K deficiency inhibited stomatal closure and A, whereas others revealed no significant effect of K on A and Gs in wall-watered growing media or promotion of stomatal opening and E by K deficiency under water deficit stress (Wang et al. 2013). These suggest that several factors may influence the effect of K on A, Gs, and E, such as plant genotype, research system, and non-manipulated environmental factors (Wang et al. 2013). In this study, K might increase under water deficit stress to prevent dramatically slowed stomatal response, increased water vapor loss, and decreased A.
The results of this experiment indicate that the effect of water deficit stress on foliar elements depends on soil fertilization and that the soil water status can modify the effect of fertilization on foliar P and K contents in Japanese larch seedlings. Specifically, DR largely decreased P in the LF treatment, but only moderately increased P in the HF, relative to WW, while HF largely enriched P content in the DR treatment but not in the WW treatment, relative to LF. These results agree with the broader literature for various tree species and experimental systems. For example, a meta-analysis revealed an average of 9.2% lower P concentration and 7.0% higher N/P ratio, across studies, with the magnitude of the effect declining from short term (< 90 d) to longer (> 90 d) (He and Dijkstra 2014). Importantly, a series of studies showed that drought facilitates conversion of soil P to forms that are not directly accessible to plants (Sardans and Peñuelas 2004) and decreases P bioavailability in different kinds of forests (Sardans and Peñuelas 2004, 2007; Zhang et al. 2020). For example, a 6-year-long study in a Mediterranean Quercus ilex forest revealed increased soil soluble organic P (and thus total soil soluble P) and smaller release of inorganic P from P bound to organic matter (Sardans and Peñuelas 2007). Another 4-year-long study in a warm temperate forest revealed that P held in Ca phosphate decreased during drought, and organic and inorganic P bound to Fe/Al oxides (secondary minerals) increased (Zhang et al. 2020). These effects could be explained by a lower soil pH under drought, which can modulate the solubilization of P held in Ca phosphate (Zhang et al. 2020). Decreased uptake of P and increased recalcitrance of elements in soil can result in reduced P (and K) in an ecosystem (Sardans and Peñuelas 2007).
The results indicated that DR increased K content in HF but not in LF, and HF enhanced K content in DR but not in WW. These findings indicate that the response of foliar K to drought prevailed only under sufficient fertilization and that the effect of HF on foliar K was promoted by drought stress. Drought affects K in soil, for example, increasing its total content by 10% and decreasing the soil soluble form by approximately 20% in a Mediterranean Q. ilex forest (Sardans and Peñuelas 2007). Some studies suggest that increased soil K can increase maximum Gs and, thus, increase water demand and enhance water stress (Battie-Laclau et al. 2014). Although increased foliar K content can improve tolerance to drought, comprehensive assessments are needed to consider growth improvement while keeping Gs and water requirements at relatively low levels to prevent water deficiency and stress during drought (Battie-Laclau et al. 2014; Santos et al. 2021).
Exposure to DR led to a moderate decrease in foliar P content compared to the WW treatment in ECM-treated plants, further suggesting soil ECM inoculation worsened the effect of drought on P status, compared to non-ECM soils. Besides, the largely enriched foliar P in ECM-treated plants (compared to non-ECM plants) under WW may indicate that ECM colonization in inoculated plants promoted access to P and its uptake by plants and/or that distinct microbial communities that developed after the inoculation increased the concentrations of bioavailable forms of P in the soil. Therefore, soil water status modulated the effect of ECM treatment on foliar P, an element that is highly driven by ECM. Based on the results of the elemental analysis, nutrient imbalance might not be caused by ECM inoculation (in WW plants), which is also supported by the lack of significant difference in A390 between ECM and non-ECM. Thus, the reduction in growth, a negative effect of ECM, might result from a consumption of photosynthates by ECM. Conversely, ECM treatment might enhance P uptake in WW plants, a positive effect of ECM. Regulation of such conflicting advantages and disadvantages of ECM treatment are important in the practical translation of scientific findings, especially when container seedlings are planted in P-deficient field conditions. Further studies are required to comprehensively evaluate such potential conflicting effects of ECM treatment.
The results of the multiple comparisons within the fertilization × ECM × irrigation interaction also provided evidence indicating that ECM, compared to non-ECM, led to decreased water potential under drought in the LF treatment, suggesting a negative effect when ECM and DR treatments were combined with the LF treatment. From a different point of view, these results may also indicate that ectomycorrhizae decreased the negative effect of DR and LF because non-ECM plants had more root tips and complete ectomycorrhizae. Further support for this suggestion is provided by the multiple comparisons within the significant ECM × irrigation interaction for the ratio of shed needles mass to all needles mass: DR largely increased the ratio relative to WW in ECM-treated plants. Specifically, the intensification of senescence by DR was exacerbated by ECM treatment or alleviated by ectomycorrhizae in non-ECM plants with more root tips and complete ectomycorrhizae. The practical significance of these effects indicates the effectiveness of DR treatment to induce water deficit stress. The absence of evidence for significant effects of DR on growth and productivity of seedlings might be due to the seedlings reaching their maximum growth in the cultivation conditions, especially because DR was applied at the beginning of October.
The findings of this study, based on modern practice of transplanting container-grown seedlings, indicate that plantations derived from container-grown seedlings might be severely affected by irregular cycles between dry and moist conditions under climate change. Although long-term field studies are needed to better understand the implications over a longer period, this study reveals some important mechanisms underlying the responses to DR, which can guide programs seeking to enhance seedling performance during droughts. In particular, fertilization practices when drought is imminent need to be reconsidered. However, we also identified fertilization regimes that can be incorporated into management practices to improve production.
Conclusions
Two grams of slow-release fertilizer per plant (HF) greatly increased growth and biomass of seedlings, favoring aboveground expansion over belowground growth. However, HF also largely increased root biomass and led to a denser root system, but resulted in smaller Gs390 and E390. Based on these outcomes, HF enhanced plant growth and productivity.
ECM inoculation produced a series of negative effects of moderate magnitude on seedling growth and biomass, irrespective of other factors, while also largely counteracting the positive effect of HF on seedling biomass and foliar K content. The ECM inoculation did not enhance seedling health and vigor, suggesting that a general “all-microbe inoculum” may not be ideal for this purpose; thus, inoculation with individually selected species of symbiotic organisms may be a better strategy. This study also indicates the importance of ectomycorrhization in producing superior seedlings because the inoculated seedlings had fewer root tips and more incomplete ectomycorrhizae and were thus inferior to the non-inoculated seedlings.
Water deficit stress (DR) greatly promoted stomatal closure, decreased CO2 assimilation, and induced needle senescence. It also greatly affected K and Mg homeostasis. Foliar K content increased and Mg content decreased, suggesting that modifying fertilization regimes to favor Mg during drought may facilitate adaptive responses and enhance the drought tolerance of Japanese larch container seedlings.
DR also moderately increased foliar P content in the presence of HF but largely decreased it in LF, suggesting a pronounced DR-induced stress in nutrient-limited conditions. Moreover, an increase in foliar K by DR occurred only under sufficient fertilization, and the effect of HF on foliar K was promoted by water deficit stress. Hence, foliar K and P response to water deficit stress was driven by soil fertility.
DR further resulted in a moderate decrease in foliar P in ECM-treated plants, suggesting that DR worsened the negative effect of soil ECM inoculation on the P status. In addition, the intensification of senescence by DR was exacerbated by the ECM treatment. Conversely, ECM treatment produced a large increase in foliar P under WW, indicating that ECM-treated plants might access more P under WW. The ECM treatment lowered the plant water potential under DR in the LF treatment, pointing to a negative effect when ECM and DR treatments are both present when plants are grown in LF.
These results lead to the conclusion that the success of the ECM inoculation depends on the soil water conditions, so future studies that include several degrees of soil wetness and different ECM inoculations are needed. Moreover, the effect of water deficit stress on container-grown Japanese larch seedlings is modulated by soil fertility. These findings reveal that innovative forestry programs are needed to test the effects of different degrees of soil wetness and frequencies and intensities of drying–rewetting cycles as a function of different fertilization regimes to enhance the health, vigor, and success of forestation seedlings in future climate changes.
This study lasted only one growing season and with a relatively short time between treatments. Hence, longer studies are needed, especially to examine whether a longer gap between experimental treatments will produce different results and if suitable fertilization and ECM inoculation regimes in one growing season can better modulate drought stress in succeeding growing seasons. Studies are also needed to investigate how the timing and duration of drought influences the effect of fertilization and ectomycorrhization on plants during a drought. Nevertheless, this study provides new integrated information that can enlighten forestry practice, offering a perspective to improve the quality of Japanese larch seedlings for forestation.
References
Agathokleous E, Belz RG, Kitao M, Koike T, Calabrese EJ (2019) Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J Forestry Res 30:1569–1580
Agathokleous E, Kitao M, Komatsu M, Tamai Y, Saito H, Harayama H, Uemura A, Tobita H, Koike T (2020) Effects of soil nutrient availability and ozone on container-grown Japanese larch seedlings and role of soil microbes. J Forestry Res 31:2295–2311
Agathokleous E, Saitanis CJ (2020) Plant susceptibility to ozone: a Tower of Babel? Sci Total Environ 703:134962
Agathokleous E, Saitanis CJ, Stamatelopoulos D, Mouzaki-Paxinou AC, Paoletti E, Manning WJ (2016) Olive oil for dressing plant leaves so as to avoid O3 injury. Water Air Soil Pollut 227:282
Ahluwalia O, Singh PC, Bhatia R (2021) A review on drought stress in plants: implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain 5:100032
Aliniaeifard S, Shomali A, Seifikalhor M, Lastochkina O (2020) Calcium signaling in plants under drought. In: Hasanuzzaman M, Tanveer M (eds) Salt and drought stress tolerance in plants. Springer, Cham, pp 259–298
Alvarez M, Huygens D, Olivares E, Saavedra I, Alberdi M, Valenzuela E (2009) Ectomycorrhizal fungi enhance nitrogen and phosphorus nutrition of Nothofagus dombeyi under drought conditions by regulating assimilative enzyme activities. Physiol Plant 136:426–436
Aryal P, Meiners SJ, Carlsward BS (2020) Ectomycorrhizae determine chestnut seedling growth and drought response. Agrofor Syst 95:1251–1260
Battie-Laclau P, Laclau JP, Domec JC, Christina M, Bouillet JP, de Cassia PM, de Moraes Gonçalves JL, Moreira RM, Krusche AV, Bouvet JM, Nouvellon Y (2014) Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytol 203:401–413
Bernardo L, Carletti P, Badeck FW, Rizza F, Morcia C, Ghizzoni R, Rouphael Y, Colla G, Terzi V, Lucini L (2019) Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol Biochem 137:203–212
Box GEP, Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B 26:211–252
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916
Chen XB, Sun XM, Dong LM, Zhang SG (2018) Mating patterns and pollen dispersal in a Japanese larch (Larix kaempferi) clonal seed orchard: a case study. Sci China Life Sci 61:1011–1023
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates
Corsini D, Vigevani I, Oggioni SD, Frangi P, Brunetti C, Mori J, Viti C, Ferrini F, Fini A (2022) Effects of controlled mycorrhization and deficit irrigation in the nursery on post-transplant growth and physiology of Acer campestre L. and Tilia cordata Mill. Forests 13:658
Dai A (2011) Drought under global warming: a review. Wires Clim Chang 2:45–65
Emery SM, Stahlheber KA, Gross KL (2020) Drought minimized nitrogen fertilization effects on bioenergy feedstock quality. Biomass Bioenergy 133:105452
Ewers BE, Oren R, Sperry JS (2000) Influence of nutrient versus water supply on hydraulic architecture and water balance in Pinus taeda. Plant Cell Environ 23:1055–1066
Guo WL, Chen SN, Hussain N, Cong YX, Liang ZS, Chen KM (2015) Magnesium stress signaling in plant: just a beginning. Plant Signal Behav 10:e992287
Guo WL, Nazim H, Liang ZS, Yang DF (2016) Magnesium deficiency in plants: an urgent problem. Crop J 4:83–91
Hao ZC, AghaKouchak A, Nakhjiri N, Farahmand A (2014) Global integrated drought monitoring and prediction system. Sci Data 1:1–10
Harayama H, Tobita H, Kitao M, Kon H, Ishizuka W, Kuromaru M, Kita K (2021) Enhanced summer planting survival of Japanese larch container-grown seedlings. Forests 12:1115
Hauer-Jákli M, Tränkner M (2019) Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: a systematic review and meta-analysis from 70 years of research. Front Plant Sci 10:766
He MZ, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol 204:924–931
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Hu Y, Chen B (2020) Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid, malic acid accumulation, and improvement of nitrogen assimilation in roots of water-stressed maize plants. Mycorrhiza 30:329–339
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Johnson R, Vishwakarma K, Hossen MS, Kumar V, Shackira AM, Puthur JT, Abdi G, Sarraf M, Hasanuzzaman M (2022) Potassium in plants: growth regulation, signaling, and environmental stress tolerance. Plant Physiol Biochem 172:56–69
Kamrani YY, Shomali A, Aliniaeifard S, Lastochkina O, Moosavi-Nezhad M, Hajinajaf N, Talar U (2022) Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 11:1154
Kitao M, Agathokleous E, Harayama H, Kitaoka S, Uemura A, Yazaki K, Tobita H (2022) Tolerance of Japanese larch to drought is modified by nitrogen and water regimes during cultivation of container seedlings. Eur J for Res 141:699–712
Kochenderfer JN, Smith HC, Crews JT (1995) Effects of fertilization on the growth and development of a Japanese larch plantation in West Virginia. Radnor, U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station
Kurinobu S (2015) Forest tree breeding for Japanese larch. Euras J for Res 8:127–134
Li JY, Wu GX, Guo QX, Korpelainen H, Li CY (2018) Fast-growing Larix kaempferi suffers under nutrient imbalance caused by phosphorus fertilization in larch plantation soil. For Ecol Manage 417:49–62
Lisar SYS, Motafakkerazad R, Hossain MM, Rahman IMM (2012) Water stress in plants: causes, effects and responses. In: Md. mofizur Rahman I, Hasegawa H (eds) Water stress. IntechOpen, Berlin, pp 1–14
Malhotra H, Vandana Sharma S, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Nowak BH (eds) Plant nutrients and abiotic stress tolerance. Springer, pp 171–190
Maltz MR, Chen Z, Cao JX, Arogyaswamy K, Shulman H, Aronson EL (2019) Inoculation with Pisolithus tinctorius may ameliorate acid rain impacts on soil microbial communities associated with Pinus massoniana seedlings. Fungal Ecol 40:50–61
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London
Masaki T, Oguro M, Yamashita N, Otani T, Utsugi H (2017) Reforestation following harvesting of conifer plantations in Japan: current issues from silvicultural and ecological perspectives. Reforesta 3:125–142
Matyssek R, Schulze E-D (1987) Heterosis in hybrid larch (Larix decidua × leptolepis). Trees 1:225–231
Pandey GK, Mahiwal S (2020) Potassium in abiotic stress. In: Pandey GK, Mahiwal S (eds) Role of potassium in plants. Springer, Cham, pp 45–49
Paoletti E, Grulke NE (2005) Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ Pollut 137:483–493
Petrov P, Petrova A, Dimitrov I, Tashev T, Olsovska K, Brestic M, Misheva S (2018) Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J Agron Crop Sci 204:219–227
Pons C, Müller C (2022) Impacts of drought stress and mycorrhizal inoculation on the performance of two spring wheat cultivars. Plants 11:2187
Qu LY, Quoreshi AM, Koike T (2003) Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant Soil 255:293–302
Qu LY, Wang Y, Masyagina O, Kitaoka S, Fujita S, Kita K, Prokushkin A, Koike T (2022) Larch: a promising deciduous conifer as an eco-environmental resource. Conifers—recent advances. IntechOpen, pp 1–37
Santos EF, Mateus NS, Rosário MO, Garcez TB, Mazzafera P, Lavres J (2021) Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol Plant 172:552–563
Sardans J, Peñuelas J (2004) Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil 267:367–377
Sardans J, Peñuelas J (2007) Drought changes phosphorus and potassium accumulation patterns in an evergreen Mediterranean forest. Funct Ecol 21:191–201
Sarraf M, Vishwakarma K, Kumar V, Arif N, Das S, Johnson R, Janeeshma E, Puthur JT, Aliniaeifard S, Chauhan DK, Fujita M, Hasanuzzaman M (2022) Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: an overview of the mechanisms. Plants 11:316
Shehab AESAE, Guo Y (2021) Effects of nitrogen fertilization and drought on hydrocyanic acid accumulation and morpho-physiological parameters of sorghums. J Sci Food Agric 101:3355–3365
Sun XG, Shi J, Ding GJ (2017) Combined effects of arbuscular mycorrhiza and drought stress on plant growth and mortality of forage sorghum. Appl Soil Ecol 119:384–391
Svenson SE, Davies FT, Meier CE (1991) Ectomycorrhizae and drought acclimation influence water relations and growth of loblolly pine. HortScience 26:1406–1409
Takenaka C, Miyahara M, Ohta T, Maximov TC (2016) Response of larch root development to annual changes of water conditions in eastern Siberia. Polar Sci 10:160–166
Tallmadge G (1977) Ideabook: the joint dissemination review panel. U.S. Dept. of Health, Education and Welfare, National Institute of Education, U.S. Office of Education, Washington
Tariq A, Pan KW, Olatunji OA, Graciano C, Li ZL, Sun F, Zhang L, Wu XG, Chen WK, Song DG, Huang D, Xue T, Zhang AP (2018) Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci Rep 8:1–11
Turcsán A, Steppe K, Sárközi E, Erdélyi É, Missoorten M, Mees G, Mijnsbrugge KV (2016) Early summer drought stress during the first growing year stimulates extra shoot growth in oak seedlings (Quercus petraea). Front Plant Sci 7:193
Ulrich DEM, Sevanto S, Ryan M, Albright MBN, Johansen RB, Dunbar JM (2019) Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci Rep 9:949
Vicente-Serrano SM, Quiring SM, Peña-Gallardo M, Yuan S, Domínguez-Castro F (2020) A review of environmental droughts: Increased risk under global warming? Earth-Science Rev 201:102953
Wang M, Zheng QS, Shen QR, Guo SW (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Wang XN, Agathokleous E, Qu LY, Fujita S, Watanabe M, Tamai Y, Mao QZ, Koyama A, Koike T (2018) Effects of simulated nitrogen deposition on ectomycorrhizae community structure in hybrid larch and its parents grown in volcanic ash soil: the role of phosphorous. Sci Total Environ 618:905–915
Wang XN, Qu LY, Mao QZ, Watanabe M, Hoshika Y, Koyama A, Kawaguchi K, Tamai Y, Koike T (2015) Ectomycorrhizal colonization and growth of the hybrid larch F1 under elevated CO2 and O3. Environ Pollut 197:116–126
Ward EJ, Domec JC, Laviner MA, Fox TR, Sun G, McNulty S, King J, Noormets A (2015) Fertilization intensifies drought stress: Water use and stomatal conductance of Pinus taeda in a midrotation fertilization and throughfall reduction experiment. For Ecol Manag 355:72–82
Wolf FM (1986) Meta-analysis: quantitative methods for research synthesis. Sage Publications, Beverly Hills
Xu ZZ, Zhou GS, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60:3737–3749
Xu ZZ, Zhou GS, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649
Yamamoto K (2003) LIA for Win 32 (LIA 32). http://www.agr.nagoya-u.ac.jp/∼shinkan/LIA32/index.html. Accessed 10 Oct 2010 (in Japanese)
Yi YM, Xie ZH, Zhang DQ, Peng JY, Huang TW, Yang LB (2002) Effect on growth response to fertilization in the young Japanese larch plantation. J Huazhong Agric Univ 21:231–234 (in Chinese)
Yin DC, Song RQ, Qi JY, Deng X (2017) Ectomycorrhizal fungus enhances drought tolerance of Pinus sylvestris var. mongolica seedlings and improves soil condition. J Forestry Res 29:1775–1788
Zhang HZ, Shi LL, Lu HB, Shao YH, Liu SR, Fu SL (2020) Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci Total Environ 732:139295
Zhuang X, Hao ZC, Singh VP, Zhang Y, Feng SF, Xu Y, Hao FH (2022) Drought propagation under global warming: characteristics, approaches, processes, and controlling factors. Sci Total Environ 838:156021
Acknowledgements
The authors are grateful to Ms. H. Yamamoto for technical assistance in the cultivation and maintenance of the seedlings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Although E.A. is Associate Editor-in-Chief of this journal, he was not involved in the peer review of this article. The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by JSPS KAKENHI Grant Number JP17F17102 (to EA and MK), Startup Foundation for Introducing Talent of Nanjing University of Information Science and Technology (NUIST) (No. 003080), and the Jiangsu Distinguished Professor program of the People’s Government of Jiangsu Province.
The online version is available at http://www.springerlink.com.
Corresponding editor: Tao Xu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agathokleous, E., Kitao, M., Komatsu, M. et al. Single and combined effects of fertilization, ectomycorrhizal inoculation, and drought on container-grown Japanese larch seedlings. J. For. Res. 34, 1077–1094 (2023). https://doi.org/10.1007/s11676-022-01565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01565-3