Abstract
This study was performed to observe the effects of water on photosynthesis and water-related physiology in dominant shrubs in shell sand habitats. Four-year-old Periploca sepium seedlings were used as model species. A gradient of 12 water levels was established by artificially supplying the shell sand with water up to saturation and then allowing natural evapotranspiration to occur. The photosynthetic, chlorophyll fluorescence and stem sap flow parameters of P. sepium were measured under a range of water conditions. The different soil water conditions were classified according to the responses of these parameters. (1) With the increase in the relative water content (RWC) of the shell sand, the parameters of leaf photosynthesis, chlorophyll fluorescence and water-related physiology in P. sepium showed significant critical responses. The net photosynthetic rate (Pn), transpiration rate (Tr), instantaneous water use efficiency (WUE), potential water use efficiency (WUEi), maximum photochemical efficiency (Fv/Fm), actual photochemical efficiency (ΦPSII) and daily accumulation of stem sap flow all increased first and then decreased with increasing RWC, but the corresponding water conditions associated with their maximum values were not the same. An RWC of 69.40% was determined to be the optimal water condition for photosynthesis and water-related physiological activity in P. sepium. At an RWC of 36.61%, the mechanism of photosynthetic inhibition in P. sepium changed from stomatal limitation to nonstomatal limitation; this was also the minimum water requirement for maintaining normal photosynthetic processes. An RWC of 50.27% resulted in the highest WUE in P. sepium, indicating that moderate drought stress increased WUE. (2) Based on the quantitative relationship between the photosynthetic parameters of P. sepium and the shell sand water gradient, the soil water availability was classified into 6 water grades. The RWC range for maintaining strong photosynthesis and high WUE in P. sepium was 63.22–69.98%. (3) Gas exchange in P. sepium was inhibited under drought and waterlogging stresses. Under these conditions, the photosynthetic electron transport chain was blocked, and the dissipation of light energy as heat increased, which ultimately led to a decline in photosynthetic productivity; moreover, transpiration and dissipation were aggravated, and water transmission and utilization processes in P. sepium were hindered. A significant negative feedback regulation mechanism in the photosynthetic and water-related physiological processes of P. sepium was observed; this mechanism allowed P. sepium growing in shell sand to be highly adaptable to water stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their high burial of groundwater and to the shell ridges in the Yellow River delta being unique, beach-like ridge landforms formed by long-term tidal erosion and transport processes deposit the remains of ancient crustaceans on the muddy coast (Xie et al. 2012; Xia et al. 2020). These shell ridges are unique arid habitats with high evaporation-rainfall ratios. Xerophytes and shrub vegetation have become the dominant species in shell ridge ecosystems (Xia et al. 2017, 2020). Soil moisture is the key factor in maintaining the functions of the dominant plant community in dry shell-sand habitats and promoting ecological succession (Zhao et al. 2015; Xia et al. 2017). Periploca sepium Bunge is a vine shrub belonging to the Periploca genus of the Asclepiadaceae family. It has good water and soil conservation and sand and drought resistance characteristics and is the main woody plant species used in the restoration of droughty shell-sand habitats (Wang et al. 2020). Plants mainly reduce the damage caused by drought stress by adjusting their water-related physiological activity, photosynthesis and other processes (Xia et al. 2017; MacAlister et al. 2020; Zhou et al. 2020). Therefore, our study can provide a research basis for an intensive analysis of the water-stress adaptation strategies of xerophyte vegetation in arid shell-sand habitats.
The amount of soil water available to plants decreases under water deficit or surplus conditions. Under these conditions, the water transmission, transpiration and dissipation processes are inhibited, and the stomata tend to close (Shen 2015; Liu et al. 2018). Stomatal closure also hinders the gas exchange process in leaves and activates the physiological oxidative stress response in plant cells, which produces a large amount of CO2. Because of the high CO2 concentration and low O2 concentration in plants, the efficiency of photosynthesis and respiration is weakened, and these processes may even stop (Liu et al. 2018; Chaeikar et al. 2020; Sim et al. 2021). The inhibition of plant photosynthesis under water stress is related to stomatal and nonstomatal limitations (Farquhar and Sharkey 1982), and the plant gas exchange process is sensitive to environmental factors. Therefore, the determination of plant gas exchange parameters and water-related physiological parameters has important research significance for measuring plant water use capacity and evaluating plant adaptation strategies to water stress. The net photosynthetic rate (Pn), transpiration rate (Tr) and water use efficiency (WUE) of Armeniaca sibirica in the loess hilly region of China (Xia et al. 2011) and Cynanchum acutum in a riparian region (El-Katony et al. 2018) were sensitive to water stress. The growth parameters of Typha latifolia in the Kissimmee wetland habitat were also significantly inhibited under harsh water conditions (Asamoah and Bork 2010). Water stress inhibits vegetative growth and photosynthesis in dominant plants in different habitats, and the degree of inhibition is related to the strength of the drought resistance of the plants. Therefore, determining the critical water threshold for plants is of guiding significance for explaining their photosynthetic and water-related physioecological characteristics under different degrees of water stress.
In recent years, related studies have focused mainly on the responses of photosynthesis and water-related physiological processes to drought stress or waterlogging stress in xerophytes under 3 to 4 water levels (Gao et al. 2017; Zhang et al. 2018; Bhusal et al. 2019; Tian et al. 2019). For example, the photosynthetic efficiency and water transmission and use efficiency of two apple varieties (Hongro and Fuji) showed a downward trend in the Daqiu Basin under drought stress, indicating that they exhibited better tolerance to drought stress (Bhusal et al. 2019). The Pn, Tr and WUE of Tamarix chinensis decreased significantly under drought stress, and the photosynthetic and water-related physiological parameters indicated that T. chinensis reduced its activity to alleviate the damage from drought stress (Gao et al. 2017). The photosynthetic mechanisms of Physocarpus amurensis and Physocarpus opulifolius were significantly inhibited in the black soil region of Northeast China under waterlogging stress, and the photosynthetic electron transport chain was damaged. The intensity of heat dissipation of luminous energy was reduced to alleviate the damage caused by water stress (Zhang et al. 2018). With the increase in waterlogging stress, the gas exchange parameters and dry matter accumulation in two hybrid varieties of spring maize (Keyu16 and Demeiya1) decreased significantly in the black soil region of Northeast China, resulting in a decrease in yield (Tian et al. 2019). The studies above focused mainly on the influence of drought or waterlogging stress on the photosynthetic efficiency of plants. However, due to the narrow water gradients involved in these studies, the specific water conditions required to maintain different degrees of photosynthetic physiological processes in plants are unclear, and it is difficult to determine the appropriate water threshold for maintaining better plant growth. There are few studies on the changes in photosynthetic and water-related physiological parameters or the ecological function of dominant shrub species under various water conditions in shell-sand habitats, and there are also few reports on the critical effects of water on plant gas exchange and water physiological processes under water stress. Thus, the response of the photosynthetic efficiency of P. sepium to the water content of shell sand and its quantitative relationship are still unclear.
To clarify these issues, this study implemented a water gradient with 12 water conditions based on the natural evapotranspiration of water from shell sand. Four-year-old seedlings of P. sepium, a typical xerophytic shrub in shell sand habitats, were taken as the research object. The photosynthetic parameters, chlorophyll fluorescence parameters and stem sap flow parameters of P. sepium were measured under different water conditions. The different soil water conditions for P. sepium were classified and evaluated on the basis of the measured photosynthetic and water-related physiological parameters. The changes in the photosynthetic and water-related physiological parameters of P. sepium were studied along a water gradient in the shell sand habitat. Determining the mechanism of the response of P. sepium to changes in water availability is expected to provide a theoretical basis for the management of water resources and the selection of suitable plants for shell sand habitats.
Materials and methods
Experimental site
The Binzhou Shell Ridge Island and Wetland National Nature Reserve is the largest existing ancient shell ridge beach in the world. It is located in Binzhou city, Shandong Province, on the southwest coast of Bohai Bay (117° 46′ 58.00″ E–118° 05′ 42.95″ E, 38° 02′ 50.51″ N–38° 21′ 06.06″ N), with an area of 43,541.54 hm2. The climate is characterized by hot and rainy conditions in summer and cold and dry conditions in winter. The terrain is relatively gentle and mainly includes coastal wetlands and shell beach ridges. Freshwater resources are relatively scarce, and the main source of water supply is atmospheric precipitation. The main vegetation types in the reserve are coastal halophyte and xerophyte communities.
Experimental materials and design
Four-year-old seedlings of P. sepium, the dominant xerophyte on the shell ridges, were used as the test material. A trunk-cutting treatment was applied uniformly to the P. sepium seedlings. The seedling height was 1.18 m, and the root diameter was 1.25 cm. The east–west, north–south crown width of the seedlings was 0.4 m. The experiment was carried out in the experimental area of Binzhou Shell Ridge Island and Wetland National Nature Reserve. The shell sand for the experiment was passed through a 2.0-mm sieve and then put into pots. To maintain consistency with the environmental conditions in the testing area, all pots were buried in shell sand. To avoid the effect of groundwater, we placed a tray under every pot to separate the shell sand in the pots from the groundwater, and the top of the pot was exposed. The pots were 40 cm in diameter and 50 cm in height. The basic physical and chemical parameters of the shell sand were as follows: field water holding capacity, 18.31%; bulk density, 1.29 g cm−3; particle size, 0.2–2.0 mm; pH, 7.40; and salt content, 0.1–0.4%.
Seedling planting began on May 10, 2017. A total of 10 pots with 3 plants in each pot were established and subjected to normal management practices. The water control treatment started on June 15, 2018. After the artificial water supply saturated the shell sand in the pots, the water gradient treatments were established by relying on plant transpiration and natural evaporation from the shell sand. To avoid the influence of rainfall on the soil moisture conditions, a simple rain shelter was built over the pots. From June 17 to July 4, the relevant indicators were monitored, and the measurements were performed on sunny days. Then, the rain shelter was removed. Five plants were randomly selected as the test objects. The gas exchange parameters, chlorophyll fluorescence indicators and stem sap flow parameters of P. sepium in the different water treatments were measured for 12 consecutive days. While monitoring the above indicators, the drying method was used to measure the water content of the shell sand by weight, and the relative water content (RWC) of the shell sand was calculated. A total of 12 RWC conditions (84.7%, 77.6%, 75.4%, 69.4%, 64.5%, 57.4%, 50.3%, 45.4%, 39.9%, 36.6%, 27.9%, 23.5%) were established.
Determination of gas exchange parameters
The gas exchange parameters of the seedlings were measured at 9:00–11:30 on clear days. Three healthy, mature leaves from the middle and upper parts of each plant were selected for measurement, and a Li-6400XT portable photosynthesis instrument (Li-Cor, Inc., Lincoln, NE, USA) was used to perform the light response measurements of gas exchange parameters. A standard 6400-02B red and blue light source was used in the leaf chamber, and photosynthetically active radiation (PAR) of 0, 40, 80, 100, 150, 200, 400, 600, 800, 1000, and 1200 µmol m−2 s−1 was applied for a total of 11 light conditions. During the measurements, the PAR was changed from high to low values along the gradient, and each light condition was maintained for 120 s, with 3 repetitions. The instrument automatically calculated and recorded the Pn, Tr, instantaneous water using efficiency (WUE = Pn/Tr), potential water using efficiency (WUEi = Pn/Gs), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and stomatal limit value (Ls). When PAR reached the optimal level, the gas exchange parameters under different water conditions were subjected to analysis.
Determination of chlorophyll fluorescence parameters
An FMS-2 portable pulse-modulated fluorometer (Hansatech, INC, UK) was used to determine the chlorophyll fluorescence parameters of P. sepium, and the samples were the same as those used to determine the photosynthetic physiological indicators as described above. After 30 min of dark adaptation, the initial fluorescence (F0) and maximum fluorescence (Fm) of the leaves were measured. After 50 min of adaptation under natural light, the steady-state fluorescence (Fs) and maximum fluorescence (Fm′) under light adaptation were measured. Then, the potential photochemical efficiency (Fv/Fm = (Fm − F0)/Fm), actual photochemical efficiency (ΦPSII = (Fm′ − Fs)/Fm′), nonphotochemical quenching coefficient (NPQ = (Fm − Fm′)/Fm′) and other parameters were calculated.
Determination of stem sap flow parameters
A heat-balance sap flow measurement system (Flow 32, Dynamax, Houston, USA) was used to continuously measure the stem sap flow rate and daily sap flow accumulation of P. sepium. SGA5 probes of different sizes (5–7 mm in diameter) were used according to the different stem diameters of the P. sepium seedlings. Standard installation was carried out according to the manufacturer’s instructions. The data collector (Delta-T Logger) automatically collected and recorded the instantaneous stem sap flow rate and measured the cumulative amount of sap flow. The collection interval was 30 min.
Statistical analysis
The right-angle hyperbolic correction model (Ye 2007) is used to simulate the photosynthetic light response process of PAR-Pn, and the model function is shown in formula (1).
where Pn is the net photosynthetic rate; I is the PAR; Ic is the light compensation point (LCP, µmol m−2 s−2); α, β, and γ are coefficients that are not related to the light intensity; α is the initial slope of the photoresponse curve at PAR = 0, which is regarded as the apparent quantum yield (AQY); and β and γ are the biologically significant light inhibition and light saturation terms, respectively.
The dark respiration rate (Rd, µmol m−2 s−2), light saturation point (LSP, µmol m−2 s−2), maximum net photosynthetic rate (Pmax, µmol m−2 s−2) and other photosynthetic parameters can be derived on the basis of formula (1), as shown in formulas (2–4)
where Im is the LSP.
where Pn (Im) is Pmax.
By performing mathematical regression analysis of the trends of some photosynthetic and water-related physiological parameters of shell sand at different water content levels, the corresponding regression equation mathematical models (fitting equations) were obtained. Then, the average values of some main indicators were calculated with the fitting equations, as shown in formula (5).
where 84.7 is the upper limit of RWC and 23.5 is the lower limit of RWC.
Data processing, mapping and fitting analyses were performed with Excel 2016. SPSS 19.0 was used to perform correlation analysis, one-way analysis of variance (ANOVA) and multiple comparisons.
Results
Response of P n and T r to the water gradient
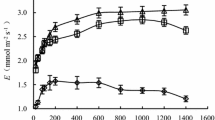
With increasing RWC in the shell sand, the Pn and Tr of P. sepium first increased and then decreased. When the RWC was 69.4%, Pn and Tr both achieved maximum values (Fig. 1A). The mathematical models of the relationships between Pn and Tr and the moisture content of shell sand are shown in formulas (6, 7).
where WR is the RWC of the shell sand soil (the same below).
From the formulas, the maximum net photosynthetic rate (Pn = 8.77 µmol m−2 s−1) and maximum transpiration rate (Tr = 2.26 mmol m−2 s−1) were calculated. The RWC values corresponding to the maximum photosynthetic rate and the maximum transpiration rate were 69.98% and 69.04%, respectively. When Pn was zero, the corresponding RWCs were 25.84% and 114.11%, and when Tr was zero, the corresponding RWCs were 25.64% and 112.44%. Of these values, 144.11% and 112.44% are over 100% and, therefore, have no biological significance, so they were not included in further analyses. An RWC of 25.84% was the water compensation point for Pn, and an RWC of 25.64% was the water compensation point for Tr. According to formula (5), the average value of Pn was 6.23 µmol m−2 s−1, and the corresponding RWCs were 46.22% and 93.73%, respectively. Similarly, the average value of Tr was 1.62 mmol m−2 s−1, and the corresponding RWCs were 45.90% and 92.18%. The RWCs for maintaining a moderate or higher Pn and Tr were 46.22–93.73% and 45.90–92.18%, respectively.
Response of WUE and WUE i to the water gradient
As the RWC of the shell sand increased, the WUE and WUEi first significantly increased (P < 0.05) and then slowly decreased (P < 0.05) (Fig. 1B). When the RWCs were 50.3% and 64.5%, WUE and WUEi reached their maximum levels, respectively. The mathematical models of the relationships between WUE and WUEi and the shell sand RWC are shown in formulas (8, 9).
From the formulas, the highest values of the instantaneous water use efficiency and the potential water use efficiency (WUE = 4.91 μmol∙mmol−1, WUEi = 0.093 μmol∙mmol−1) were calculated, and the RWCs corresponding to the highest values of WUE and WUEi were calculated to be 63.22% and 61.67%, respectively. When the WUE was zero, the corresponding RWCs were 18.90% and 107.54%, and when the WUEi was zero, the corresponding RWCs were 22.28% and 101.05%. Of these values, 107.54% and 101.05% are over 100% and therefore have no biological significance, so they were not included in further analyses. RWCs of 18.90% and 22.28% were determined to be the water compensation points for WUE and WUEi, respectively. According to formula (5), the average values of WUE and WUEi were determined to be 3.92 and 0.071 μmol mmol−1, respectively. The RWCs corresponding to the average WUE were 43.34% and 83.10%, and the RWCs corresponding to the average WUEi were 42.45% and 80.89%. The RWCs that maintained WUE and WUEi above moderate levels were 43.34–83.10% and 42.45–80.89%, respectively.
Response of photosynthetic light response parameters to the water gradient
With increasing shell sand RWC values, AQY, Rd (Fig. 2A) and Pmax (Fig. 2C) first increased significantly (P < 0.05) and then decreased significantly (P < 0.05), but Pmax did not change significantly (P > 0.05) under severe drought stress (RWCs of 23.5–45.4%). All three parameters reached maximum values at an RWC of 69.4% (AQY = 0.0326 mol·mol−1, Rd = 1.99 µmol m−2 s−1 and Pmax = 10.087 µmol m−2 s−1).
The LCP and LSP showed diametrically opposite changes (Fig. 2B). When the RWC was 23.5–36.6%, the LCP was significantly decreased (P < 0.05) and the LSP was significantly increased (P < 0.05). When the RWC was 36.6–75.4%, the LCP remained low (P > 0.05) while the LSP reached a stable, higher value (P > 0.05). When the RWC exceeded 75.4%, the LCP began to increase significantly (P < 0.05), and the LSP decreased significantly (P < 0.05). At RWC values of 64.5–69.4%, the ability of P. sepium to use light energy was the strongest and its ecological amplitude for light was the largest. Above or below this RWC range, the light energy utilization capacity of P. sepium was significantly inhibited (P < 0.05), and its ecological amplitude for light decreased.
Response of stomatal limitation parameters to the water gradient
At RWC values of 23.5–69.4%, Gs was significantly higher than at other RWC values (P < 0.05), and Gs reached its maximum value at an RWC of 69.4%. At higher RWC values (69.4–84.7%), it decreased significantly (P < 0.05) (Fig. 3A).
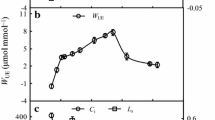
With increasing shell sand RWCs, Ci and Ls showed diametrically opposite changes (Fig. 3B). When the RWC was 23.5–36.6%, the Ci significantly decreased (P < 0.05), and Ls increased significantly (P < 0.05). When the RWC was 36.6%, Ci reached a minimum and Ls reached a maximum. Thereafter, as the moisture level increased (the RWC was 36.6–57.4%), Ci began to increase (P < 0.05) and Ls decreased significantly (P < 0.05). When the RWC increased from 57.4 to 75.4%, Ci and Ls increased and then stabilized (P > 0.05); when the RWC exceeded 75.4%, Ci decreased significantly (P < 0.05) and Ls increased significantly (P < 0.05). Therefore, an RWC of 36.6% is the critical point at which the photosynthetic efficiency of P. sepium leaves is restricted by the stomata. Below this water threshold, the photosynthetic mechanism is damaged; an RWC of 57.4–75.4% corresponds to the active moisture range of the maximum photosynthetic physiological activity in leaves.
Water availability classes of the shell sand corresponding to photosynthetic parameters
The shell sand moisture thresholds corresponding to the highest, lowest and average values of Pn and WUE were used as the critical points of water availability and were considered together with the response relationships between Pn and WUE and RWC. These thresholds were used to classify the moisture availability in shell sand with regard to photosynthetic parameters (Table 1). Production was defined as the Pn, and efficiency was defined as the WUE. The level of production efficiency was determined by the values of Pn and WUE under different shell sand moisture conditions. The RWC value at which Pn was 0, i.e., 25.84%, was considered the “water compensation points of Pn”. Therefore, RWC values below this moisture point were considered “nonproductive water”. The RWC value at which the mechanism of the decrease in Pn changed from stomatal limitation to nonstomatal limitation, i.e., 36.6%, was considered the “Pn stomatal limitation turning point”. The RWC values at which Pn and WUE reached their highest levels, i.e., 69.98% and 63.22%, respectively, were considered the “Pn saturation point” and the “maximum WUE point”. Therefore, the RWC range defined as “high-productivity, high-efficiency water conditions” was 63.22–69.98%. “Moderate-productivity, moderate-efficiency water conditions” were defined according to the RWCs when the Pn and WUE reached their average values and the highest-efficiency RWC values for Pn and WUE; these RWC ranges were 46.22–63.22% and 69.98–83.10%, respectively. The RWC range between moderate productivity and no productivity was defined as “low-productivity, low-efficiency water conditions”. As shown in Table 1, the productivity and efficiency levels were specifically divided into six classes of water availability in shell sand: nonproductive, ineffective water conditions; low-productivity, low-efficiency water conditions; low-productivity, moderate-efficiency water conditions; moderate-productivity, moderate-efficiency water conditions; moderate-productivity, low-efficiency water conditions; and high-productivity, high-efficiency water conditions.
Response of chlorophyll fluorescence parameters to the water gradient
When the RWC was 23.5–36.6%, the F0 and NPQ were significantly reduced compared with those at other RWC levels (P < 0.05). When the RWC was 36.6–69.4%, it continued to decrease but at a slower rate. When the RWC was 69.4%, both F0 and NPQ reached minimum values (142.28 and 0.568, respectively). Finally, when the RWC exceeded 69.4%, both F0 and NPQ began to increase significantly (P < 0.05) (Fig. 4A, C).
With increasing shell sand RWC, Fv/Fm, ΦPSII, and Fm first increased and then decreased (Fig. 4A, B). When the RWC of the shell sand was 23.5–36.6%, all three parameters increased significantly (P < 0.05). When the RWC exceeded 36.6%, ΦPsII and Fm continued to increase (P < 0.05) while Fv/Fm remained high, but the change was not significant (P > 0.05). When the RWC reached 50.27%, Fm stopped increasing and remained high, and the change was not significant (P > 0.05); ΦPSII continued to increase (P < 0.05). When the RWC was 69.40%, all three parameters achieved their maximum values (Fv/Fm: 0.8692, ΦPSII: 0.7602 and Fm: 1515.2). When the RWC exceeded 77.60%, Fv/Fm, ΦPSII and Fm all began to decrease significantly (P < 0.05).
Response of stem sap flow parameters to the water gradient
With increasing shell sand RWC, the daily dynamic trend of the stem sap flow rate showed a multiple-peak pattern (Fig. 5A). The runtime, peak instantaneous stem sap flow rate and daily amplitude all showed a trend of first increasing and then decreasing. The instantaneous stem sap flow rate during the day was significantly higher than that at night (P < 0.05). Under severe drought stress in the shell sand (RWC < 36.6%), little sap flow occurred at night or in the early morning. The instantaneous stem sap flow rate and daily amplitude showed decreasing trends at low RWC values (< 36.6%) and at high RWC values (> 75.4%). However, the overall trend of the instantaneous stem sap flow rate at high moisture levels was significantly higher than that at low moisture levels. At other moisture levels, the instantaneous rate and daily amplitude of the stem sap flow remained relatively high. When the RWC was 69.4%, the daily runtime, instantaneous sap flow rate and daily sap flow amplitude all reached maximum values.
With increasing shell sand RWC, significant differences in the daily stem sap flow accumulation were observed (P < 0.05). The sap flow activity during the day was more vigorous than that at night (Fig. 5B). At RWC values of 23.5%, 39.9% and 77.6%, the daily cumulative change in the stem sap flow showed a significant “S-shaped” trend. The stem sap flow accumulation from 0:00 to 6:30 was almost 0 but began to increase rapidly afterward. At approximately 15:00, the stem sap flow accumulation reached its highest value at an RWC of 23.5%. At RWCs of 39.9% and 77.6%, the stem sap flow accumulation reached its highest level after 17:00. Afterward, the sap flow accumulation remained basically stable, and sap flow tended to stop. At other RWC values, the daily cumulative change in the stem sap flow exhibited a linear trend. Overall, the daily stem sap flow accumulation process showed a rapidly increasing trend throughout the day. However, at RWC levels of 64.5%, 75.4% and 84.7%, there was a stable turning point at approximately 10:30, after which the sap flow accumulation increased rapidly. The analysis of the total daily sap flow accumulation revealed the following results. With increasing shell sand RWC, the daily cumulative sap flow first increased and then decreased. An RWC of 69.4% was the point of the most active daily accumulation (a daily cumulative sap flow of 220.6 g d−1). The daily cumulative sap flow at high moisture levels (RWC > 75.4%) was higher than that under severe drought stress (RWC < 36.6%).
Discussion
Effect of the shell sand water gradient on the photosynthetic parameters of P. sepium
As the water deficit in the shell sand increased, the light energy utilization capacity of the leaves of P. sepium decreased, and its ecological amplitude for light narrowed; these conditions resulted in a significant inhibition of photosynthetic productivity and a decrease in respiratory efficiency. The AQY range of plant leaves is 0.03–0.05 mol mol−1 under typical natural conditions (Xia et al. 2011). This range is higher than that determined in the leaves of P. sepium under water stress in the shell sand in this study, indicating that P. sepium living on shell ridges have a low adaptive capacity for low light-intensity conditions. The LSP range of plants that prefer full-sun conditions is > 600 μmol m−2 s−1, and their LCP is maintained at 14–40 μmol m−2 s−1 (Xia et al. 2011). The LSP of P. sepium on shell ridges determined in this study was generally within this range, and the LCP was mostly higher than the normal range for sun-tolerant plants. These results indicate that P. sepium on shell ridges has the same LCP and LSP values as other sun-tolerant plants, a broad ecological amplitude for light, and a strong capacity to use light energy. When the RWC was 69.40%, P. sepium had the most active photosynthesis at this RWC level. Therefore, this soil moisture level is the optimal level for photosynthetic activity. The photosynthetic parameters of P. sepium in the loess hilly area of China (An et al. 2010), Ziziphus jujuba on shell ridges (Wang et al. 2013), Forsythia suspensa in rocky mountain soil of China (Lang et al. 2018) and two apple cultivars in the Daegu basin area (Bhusal et al. 2020) all decrease to different extents under drought or waterlogging stress. However, the optimal water conditions for these plants are obviously different because of the different photosynthetic physiological regulation mechanisms adopted by the different plants under water stress in their different habitats. The response trends of the photosynthetic parameters of P. sepium to water stress can reflect the sensitivity of plant photosynthetic activity to water stress (McGee et al. 2021). Therefore, effective soil water utilization by plants can be evaluated on the basis of the change trends of the photosynthetic physiological processes of the plants under drought stress.
In this study, the quantitative relationships between the studied plant photosynthetic parameters and soil water conditions were established, and the soil water availability levels were graded and evaluated according to the corresponding minimum, maximum and average values of the photosynthetic parameters and the abovementioned relationships. Classifications of water availability levels based on the corresponding plant photosynthetic parameters can be used to determine the range of plant tolerance to water stress (Wang et al. 2013; Xia et al. 2017). Table 2 shows the different soil water availability levels classified by the photosynthetic productivity of several plants in different habitats (Wang et al. 2013; Xia et al. 2013; Yang 2018). The ranges of water availability in Table 2 are quite different. However, severe drought stress consistently represents nonproductive and ineffective water conditions. Low-productivity, low-efficiency water conditions are represented mostly by two ranges of water availability, namely, drought stress and high-water conditions. The range of moderate-productivity, moderate-efficiency water conditions is wide, but the range of high-productivity, high-efficiency water conditions is narrow. P. sepium on shell ridges and Z. jujuba in the central mountainous area of Shandong Province (Yang 2018) require relatively similar conditions in the high-yield, high-efficiency water conditions (Table 2). The water range for Z. jujuba on shell ridges (Wang et al. 2013) is the widest, but the values of the water range for Salix matsudana on shell ridges (Xia et al. 2013) are higher. It is suggested that P. sepium on shell ridges (RWC 63.22–69.98%) and Z. jujuba in the central mountainous area of Shandong Province (RWC 63.3–68.9%) have moderate water requirements. The ecological amplitude for water of Z. jujuba on shell ridges is the widest of the species considered in this analysis. The water range for maintaining better growth and photosynthetic activity in P. sepium on shell ridges is significantly lower than that for S. satudana. The drought resistance of P. sepium is better than that of S. montana, but both have lower drought resistance than Z. jujuba.
Analysis of stomatal limitation of photosynthesis
A decline in stomatal conductance hinders gas exchange and water dissipation in leaves. Therefore, stomatal limitation is the main mechanism for the reduction in plant photosynthetic efficiency under soil water stress (Gago et al. 2020). When the RWC of shell sand was 36.61–57.38% or 75.40–84.70%, the Gs and Ci of P. sepium leaves significantly decreased, while Ls increased significantly. These results indicate that strategic stomatal closure in P. sepium under these conditions prevented CO2 from entering the leaves, which reduced the availability of raw materials for photosynthesis, reduced the intercellular CO2 concentration and suppressed photosynthetic production. According to the study of Farquhar (Farquhar and Sharkey 1982), photosynthesis under these conditions was reduced due to stomatal restriction. When the RWC of the shell sand was 23.50–36.61%, Gs and Ls were significantly reduced, while Ci was significantly increased. Severe water deficit conditions caused damage to the photosynthetic physiological structure of plants and inhibited the opening of stomata. Under these conditions, a large amount of CO2 produced inside the leaf accumulates in the intercellular space, leading to a sharp increase in Ci. In addition, the photosynthetic capacity of the plant is severely inhibited, and the reduction in photosynthesis in plant leaves of P. sepium is manifested as nonstomatal limitation (Farquhar and Sharkey 1982). In this study, an RWC of 36.61% was the water critical point at which the reduction in photosynthesis was manifested by both stomatal limitation and nonstomatal limitation. Gs showed a very significant positive correlation with Pn, AQY, LSP and Rd (P < 0.01) and a very significant negative correlation with LCP (P < 0.01), while Ci and Ls showed a very significant negative correlation with each other (P < 0.01) (Table 3). These results indicate that stomatal factors are the main reason for the inhibition of the photosynthetic physiological processes of P. sepium under drought or waterlogging stress. Moreover, plants exhibit numerous oxidative stress responses that are activated by enzyme catalysis to alleviate the damage to the photosynthetic structure caused by drought or waterlogging stress. A large amount of CO2 accumulates in the intercellular space. Under mild drought stress, photosynthetic and respiratory physiological activities can still be maintained, and CO2 does not accumulate. Under severe drought stress, due to disruptions in photosynthetic and respiratory processes, plants cannot maintain normal photosynthetic or respiratory physiological activity, resulting in a large amount of CO2 accumulating in the intercellular space (Xia et al. 2011; Barickman et al. 2020). High-CO2 conditions significantly inhibit photosynthesis in plants, probably because the decrease in stomatal conductance at this time leads to a decrease in carbon accumulation in the plant body (De Roo et al. 2020). Therefore, plants can maintain their life activities under drought stress by reducing the intensity of photosynthesis and respiratory consumption and accumulating water and dry matter (Avila-Lovera et al. 2020; Thomas et al. 2020). This may be the chief reason why the photosynthetic efficiency of the leaves of P. sepium decreased under drought or waterlogging stress. Photosynthetic activity in Populus × euramericana (Liang et al. 2019), Z. jujuba on shell ridges (Wang et al. 2013), common reed in the Hexi Corridor (Zhang et al. 2019), F. suspensa in rocky mountain soil of China (Lang et al. 2018) and tung oil seedlings in South China (Li et al. 2017) was shown to be inhibited under water stress by stomal limitation, and their photosynthetic parameters showed a significant water threshold effect. The critical points of soil moisture at which the change in stomatal limitation occurred in different plants were as follows: Z. jujuba on shell ridges (25%) < P. sepium (36.61%) < F. suspensa (37.52%) < Populus × euramericana (42%) < tung oil seedlings (50%) < common reed (75%). This comparison suggests that the ecological amplitude of P. sepium is relatively wide with respect to its drought stress tolerance, and it adapts well to drought-prone shell ridge habitats.
Effect of the shell sand water gradient on chlorophyll fluorescence parameters in P. sepium
Within the shell sand water gradient, the F0 and NPQ of the leaves of P. sepium showed similar trends. Water deficit and water saturation both cause photosynthetic productivity to decrease. However, structural damage is not caused by moderate drought stress or high-water conditions. According to stomatal limitation theory, photosynthesis decreases under these conditions due to stomatal limitation. However, the photosynthetic physiological structure of plants is severely damaged under severe drought stress. The amount of effective light energy participating in photosynthesis decreases greatly, and the amount of light energy dissipated as heat increases significantly. Eventually, photosynthesis is severely inhibited. The decline in photosynthesis under these conditions manifests as nonstomatal limitation. F0 and NPQ showed a very significant negative correlation with Pn, Gs, AQY, LSP, and Rd (P < 0.01) and a very significant positive correlation with LCP (P < 0.01, Table 3). The photosynthetic physiological process in P. sepium was severely inhibited under drought and waterlogging stresses due to the inhibition or even elimination of photoreaction center activity. Fm, Fv/Fm, and ΦPSII showed the opposite trends with F0 and NPQ (Table 3), indicating that the photosynthetic electron transfer process was hindered under drought and waterlogging stresses. Intensified photoinhibition was the main reason for the decline in photosynthetic physiological activity, which was related to the inhibition of leaf gas exchange processes (Ueno et al. 2020). The chlorophyll fluorescence parameters of Phaseolus vulgaris (Mathobo et al. 2017), Juglans regia (Liu et al. 2019), two clone varieties of Hevea brasiliensis (Falqueto et al. 2017) and Carex schmidtii (Zhang et al. 2019) were sensitive to water stress, and they all showed the same trends as the chlorophyll fluorescence parameters of P. sepium. However, the optimal moisture conditions for these fluorescence parameters varied. It has been shown that the photosynthetic physiological process in plants can be inhibited under water stress by the blockage of the photosynthetic electron transport chain (Zhuang et al. 2020), but the extent to which water stress regulates the photosynthetic physiological functions of plants differs between plant species. This may be due to the different sensitivities of substrates and enzymes related to the photosynthetic electron transfer process to water stress in different plant species, which triggers enzymatic reactions of different intensities (Wu et al. 2018).
Effect of the shell sand water gradient on water-related physiological activities in P. sepium
The peak instantaneous rate, daily variation, and daily accumulation of stem sap flow and the Tr, WUE, and WUEi of P. sepium were all sensitive to water stress in the shell sand, and their values generally first increased and then decreased with increasing RWC. Under water stress, the decrease in stomatal conductance inhibited transpiration and dissipation processes in P. sepium, which led to a decline in the Tr. Water transmission and utilization processes are blocked, water-related physiological activity is inhibited, and water-related physiological mechanisms may be damaged under drought stress (Arndt et al. 2001; Huber et al. 2015). The daily accumulation of sap flow, Tr, WUE, and WUEi all had a very significant positive correlation with Pn, Gs, AQY, LSP, and Rd (P < 0.01), and there was also a very significant positive correlation among the daily accumulation of sap flow, Tr, WUE, and WUEi (P < 0.01, Table 3). Gas exchange processes not only affected photosynthetic physiological processes in P. sepium but also affected its water-related physiological activity. Of these processes, there were significant interactions between water transportation, utilization and evapotranspiration (Chaturvedi et al. 2019). When the RWC was 69.40%, the water-related physiological activity of P. sepium was the most vigorous at this RWC level; however, the water level corresponding to the maximum values of WUE and WUEi was not the same as the optimum water level for water-related physiological activities. Both WUE and WUEi achieved maximum values under moderate drought stress, and the water condition corresponding to the maximum value of WUE (an RWC of 50.27%) was less than that corresponding to the maximum value of WUEi (an RWC of 64.52%). The effect of stomatal conductance on the water use efficiency of P. sepium may lag significantly behind its effect on transpiration. Thus, transpiration dissipation is the factor that most directly affects the water use capacity of P. sepium (Huang et al. 2020). There was a significant cubic relationship between water stress and stomatal limitation parameters. The blockage of gas exchange significantly inhibits the water transmission and utilization capacity of plants, resulting in a significant decrease in the water content and water potential of plants; therefore, plants suppress their water-related physiological activities to maintain consistent life activities (Huang et al. 2020; Abdalla et al. 2021). This may be the strategy used by P. sepium to adapt to water stress. Significant decreases in the daily accumulation of sap flow and the daily variation in sap flow in Dracaena cinnabari emerged under drought stress; these decreases alleviated the damage to plant organs caused by water stress by increasing the horizontal transportation of water (Nadezhdina et al. 2018). This finding may explain why the functions of P. sepium organs were not significantly affected by mild water stress. Therefore, P. sepium growing in shell ridge habitats exhibits strong water utilization regulation and the ability to adapt to different water conditions.
Conclusions
Within the range of the shell sand water gradient to reduce the influence of stomatal restriction and avoid damage to the photosynthetic electron transport chain, the leaves of P. sepium weaken the photosynthetic production process and increase the amount of light energy dissipated as heat. At the same time, stomatal limitation forces water loss through transpiration, and the ability of P. sepium to transport and utilize water is reduced. Adopting the abovementioned photoprotective measures and weakening water-related physiological activities allow P. sepium to alleviate the damage from water stress.
The photosynthetic and water physiological processes of P. sepium show a significant water-critical effect. An RWC of 36.6% is the lower limit of the water demand threshold for photosynthesis and water physiological activities in P. sepium, and an RWC range of 63.22–69.98% is optimal for maintaining high Pn and WUE in P. sepium. Within this range, an RWC of 69.40% is the optimum water level for photosynthesis and water-related physiological activities, while WUE reaches its optimum level under moderate drought stress (RWC of 63.22%).
In conclusion, P. sepium has a strong tolerance to water stress, a wide ecological amplitude for water, and good plasticity under varying water conditions. Therefore, this study also provides evidence supporting the exploration of the mechanism of photosynthetic physiology and water-related physiology of P. sepium under water stress. The possibility of exploring the water stress adaptation strategies of dominant xerophytes in the unique habitat of shell sand is provided.
Abbreviations
- P n :
-
Net photosynthesis rate
- PAR :
-
Photosynthetically active radiation
- T r :
-
Transpiration rate
- WUE:
-
Water use efficiency
- WUE i :
-
Potential water use efficiency
- AQY :
-
Apparent quantum yield
- LCP :
-
Light compensation point
- LSP :
-
Light saturation point
- P max :
-
Maximum net photosynthesis rate
- R d :
-
Dark respiration rate
- G s :
-
Stomatal conductance
- C i :
-
Intercellular CO2 concentration
- L s :
-
Stomatal limit value
- F 0 :
-
Initial fluorescence
- F m :
-
Maximum fluorescence
- F s :
-
Steady-state fluorescence
- F m':
-
Maximum fluorescence under light-adapted conditions
- F v/F m :
-
Potential photochemical efficiency
- Φ PSII :
-
Actual photochemical efficiency
- ETR :
-
Noncyclic photosynthetic electron transport rate
- NPQ :
-
Nonphotochemical quenching coefficient
References
Abdalla M, Carminati A, Cai GC, Javaux M, Ahmed MA (2021) Stomatal closure of tomato under drought is driven by an increase in soil-root hydraulic resistance. Plant Cell Environ 44:425–431
An YY, Hao WF, Gong CM, Han RL, Liang ZS (2010) Effects of drying and re-watering on the photosynthesis and active oxygen metabolism of Periploca sepium seedlings. Ying Yong Sheng Tai Xue Bao 21:3047–3055 (in Chinese)
Arndt SK, Clifford SC, Wanek W, Jones HG, Popp M (2001) Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol 21:705–715
Asamoah SA, Bork EW (2010) Drought tolerance thresholds in cattail (Typha latifolia): a test using controlled hydrologic treatments. Wetlands 30:99–110
Avila-Lovera E, Garcillan PP, Silva-Bejarano C, Santiago LS (2020) Functional traits of leaves and photosynthetic stems of species from a sarcocaulescent scrub in the southern Baja California Peninsula. Am J Bot 107:1410–1422
Barickman TC, Ku KM, Sams CE (2020) Differing precision irrigation thresholds for kale (Brassica oleracea L. var. acephala) induces changes in physiological performance, metabolites, and yield. Environ Exp Bot 180:104253
Bhusal N, Han SG, Yoon TM (2019) Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci Hortic Amst 246:535–543
Bhusal N, Kim HS, Han SG, Yoon TM (2020) Photosynthetic traits and plant-water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ Exp Bot 176:104–111
Chaeikar SS, Marzvan S, Khiavi SJ, Rahimi M (2020) Changes in growth, biochemical, and chemical characteristics and alteration of the antioxidant defense system in the leaves of tea clones (Camellia sinensis L.) under drought stress. Sci Hortic Am 265:109257
Chaturvedi AK, Surendran U, Gopinath G, Chandran KM, Anjali NK, Fasil CTM (2019) Elucidation of stage specific physiological sensitivity of okra to drought stress through leaf gas exchange, spectral indices, growth and yield parameters. Agric Water Manag 222:92–104
De Roo L, Lauriks F, Salomon RL, Oleksyn J, Steppe K (2020) Woody tissue photosynthesis increases radial stem growth of young poplar trees under ambient atmospheric CO2 but its contribution ceases under elevated CO2. Tree Physiol 40:1572–1582
El-Katony TM, Khedr AHAF, Mergeb SO (2018) Drought stress affects gas exchange and uptake and partitioning of minerals in swallowwort (Cynanchum acutum L.). Rend Lincei Sci Fis 29:23–34
Falqueto AR, Da-Silva RA, Gomes MTG, Martins JPR, Silva DM, Partelli PL (2017) Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Sci Hortic Ams 224:238–243
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Gago J, Daloso DM, Carriqui M, Nadal M, Morales M, Araujo WL, Nunes-Nesi A, Perera-Castro AV, Clemente-Moreno MJ, Flexas J (2020) The photosynthesis game is in the “inter-play”: mechanisms underlying CO2 diffusion in leaves. Environ Exp Bot 178:104174
Gao Y, Xia JB, Chen YP, Zhao YY, Kong QX, Lang Y (2017) Effects of extreme soil water stress on photosynthetic efficiency and water consumption characteristics of Tamarix chinensis in China’s Yellow River Delta. J Forestry Res 28:491–501
Huang Z, Liu Y, Tian FP, Wu GL (2020) Soil water availability threshold indicator was determined by using plant physiological responses under drought conditions. Ecol Indic 118:106740
Huber K, Vanderborght J, Javaux M, Vereecken H (2015) Simulating transpiration and leaf water relations in response to heterogeneous soil moisture and different stomatal control mechanisms. Plant Soil 394:109–126
Lang Y, Wang M, Xia JB, Zhao QK (2018) Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa. J For Res 29:45–53
Li Z, Tan XF, Lu K, Zhang L, Long HX, Lyu JB, Lin Q (2017) Influence of drought stress on the growth, leaf gas exchange, and chlorophyll fluorescence in two varieties of tung tree seedlings. Acta Ecol Sin 37:1515–1524 (in Chinese)
Liang GT, Bu JW, Zhang SY, Jing G, Zhang GC, Liu X (2019) Effects of drought stress on the photosynthetic physiological parameters of Populus × euramericana “Neva.” J For Res 30:409–416
Liu BB, Li M, Li QM, Cui QQ, Zhang WD, Ai XZ, Bi HG (2018) Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 56:942–952
Liu BH, Liang J, Tang GM, Wang XF, Liu FC, Zhao DC (2019) Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci Hortic Am 250:230–235
MacAlister D, Muasya AM, Crespo O, Ogola JBO, Maseko S, Valentine AJ, Ottosen CO, Rosenqvist E, Chimphango SBM (2020) Stress tolerant traits and root proliferation of Aspalathus linearis (Burm.f.) R. Dahlgren grown under differing moisture regimes and exposed to drought. S Afr J Bot 131:342–350
Mathobo R, Marais D, Steyn JM (2017) The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agr Water Manage 180:118–125
McGee T, Shahid MA, Beckman TG, Chaparro JX, Schaffer B, Sarkhosh A (2021) Physiological and biochemical characterization of six Prunus rootstocks in response to flooding. Environ Exp Bot 183:104368
Nadezhdina N, Al-Okaishi A, Madera P (2018) Sap flow measurements in a Socotra dragon’s blood tree (Dracaena cinnabari) in its area of origin. Trop Plant Biol 11:107–118
Shen JR (2015) The structure of Photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Sim YS, Yim SH, Choo YS (2021) Photosynthetic and physiological characteristics of the evergreen Ligustrum japonicum and the Deciduous Cornus officinalis. J Plant Biol 64:73–85
Thomas A, Yadav BK, Simunek J (2020) Root water uptake under heterogeneous soil moisture conditions: an experimental study for unraveling compensatory root water uptake and hydraulic redistribution. Plant Soil 457:421–435
Tian LX, Li J, Bi WS, Zuo SY, Li LJ, Li WL, Sun L (2019) Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) Under field conditions. Agr Water Manage 218:250–258
Ueno Y, Shimakawa G, Aikawa S, Miyake C, Akimoto S (2020) Photoprotection mechanisms under different CO2 regimes during photosynthesis in a green alga Chlorella variabilis. Photosynth Res 144:397–407
Wang RR, Xia JB, Yang JH, Liu JT, Zhao YY, Sun JK (2013) Response characteristics of photosynthetic and physiological parameters in Ziziphus jujuba var. spinosus seedling leaves to soil water in sand habitat formed from seashells. Acta Ecol Sin 33:6088–6096 (in Chinese)
Wang X, Xia JB, Cao XB (2020) Physiological and ecological characteristics of Periploca sepium Bunge under drought stress on shell sand in the yellow river delta of China. Sci Rep 10:9567
Wu YJ, Yun C, Tian Y, Zha TS, Liu P, Bai YJ, Ma JY, Lai ZR, Bourque CPA (2018) Photosynthetic gas-exchange and PSII photochemical acclimation to drought in a native and non-native xerophytic species (Artemisia ordosica and Salix psammophila). Ecol Indic 94:130–138
Xia JB, Zhang GC, Sun JK, Liu X (2011) Threshold effects of photosynthetic and physiological parameters in Prunus sibirica to soil moisture and light intensity. Chin J Plant Ecol 35:322–329 (in Chinese)
Xia JB, Zhang SY, Zhao ZG, Zhao YY, Gao Y, Gu GY, Sun JK (2013) Critical effect of photosynthetic efficiency in Salix matsudana to soil moisture and its threshold grade in shell ridge island. Chin J Plant Ecol 37:851–860 (in Chinese)
Xia JB, Zhao XM, Ren JY, Lang Y, Qu FZ, Xu H (2017) Photosynthetic and water physiological characteristics of Tamarix chinensis under different groundwater salinity conditions. Environ Exp Bot 138:173–183
Xia JB, Ren RR, Chen YP, Sun J, Zhao XM, Zhang SY (2020) Multifractal characteristics of soil particle distribution under different vegetation types in the Yellow River Delta chenier of China. Geoderma 368:114311
Xie WJ, Zhao YY, Zhang ZD, Liu Q, Xia JB, Sun JK, Tian JY, Sun TQ (2012) Shell sand properties and vegetative distribution on shell ridges of the Southwestern Coast of Bohai Bay. Environ Earth Sci 67:1357–1362
Yang R (2018) Effect of soil drought stress degree and duration on photosynthesis of Ziziphus jujuba. Master degree, Shandong Agricultural University, Tai’an in Chinese
Ye ZP (2007) A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 45:637–640
Zhang HH, Feng P, Yang W, Sui X, Li X, Li W, Zhang RT, Gu SY, Xu N (2018) Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J For Res 29:1049–1059
Zhang RD, Zhou YF, Yue ZX, Chen XF, Cao X, Xu XX, Xing YF, Jiang B, Ai XY, Huang RD (2019) Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 57:1076–1083
Zhao YY, Hu XM, Liu JT, Lu ZH, Xia JB, Tian JY, Ma JS (2015) Vegetation pattern in shell ridge island in China’s Yellow River Delta. Front Earth Sci 9:567–577
Zhou WG, Chen F, Meng YJ, Chandrasekaran U, Luo XF, Yang WY, Shu K (2020) Plant waterlogging/flooding stress responses: from seed germination to maturation. Plant Physiol Bioch 148:228–236
Zhuang J, Wang YL, Chi YG, Zhou L, Chen JJ, Zhou W, Song J, Zhao N, Ding JX (2020) Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. Peer J 8:10046
Acknowledgements
We are grateful to the staff of the Binzhou National Shell Ridge and Wetland Nature Reserve for providing permission to access the experimental sites and for helping with the field sample collections.
Funding
The work was supported by the Forestry Science and Technology Innovation Project of Shandong Province (No. 2019LY006), the National Natural Science Foundation of China (No. 31770761), Open Research Fund Program of Shandong Key Laboratory of Eco-Environmental Science for Yellow River Delta (Binzhou University) (No. 2020KFJJ03), and the Taishan Scholars Program of Shandong Province, China (No. TSQN201909152).
Author information
Authors and Affiliations
Contributions
XW, the first author of the paper, had overall responsibility for the experimental design, data collection, analysis, writing and project management. JX is the corresponding author and made significant contributions to the experimental setup and manuscript preparation. MD, XX, XZ and YF assisted the first author in designing the test and processing the data. QF and ZL made significant contributions to the experimental design and manuscript preparation.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the Forestry Science and Technology Innovation Project of Shandong Province (No. 2019LY006), the National Natural Science Foundation of China (No. 31770761), Open Research Fund Program of Shandong Key Laboratory of Eco-Environmental Science for Yellow River Delta (Binzhou University) (No. 2020KFJJ03), and the Taishan Scholars Program of Shandong Province, China (No. TSQN201909152).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Xia, J., Zhao, X. et al. Photosynthetic and water-related physiological characteristics of Periploca sepium in response to changing soil water conditions in a shell sand habitat. J. For. Res. 34, 453–467 (2023). https://doi.org/10.1007/s11676-022-01494-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01494-1