Abstract
Cinnamomum camphora (L.) J. Presl. (Laurales: Lauraceae) is widely cultivated as an important landscape tree species in many urban areas in South China, especially in Shanghai City. Pagiophloeus tsushimanus Morimoto has become a destructive insect pest of C. camphora plantations in Shanghai, but the biological and ecological traits of this pest remain largely unknown. In this study, we investigated the damage and life history and determined the larval instar of P. tsushimanus. The results indicated that P. tsushimanus is a monophagous weevil pest, and C. camphora is the unique host tree species. C. camphora plantations in all administrative districts of Shanghai have been seriously damaged by P. tsushimanus. Adults often aggregate for feeding on the tender bark of twigs and occasionally on newly emerged buds. After experiencing damage, the twigs shrink and crack and the buds will shrink. Adults tend to repeatedly mate and oviposit, and all females lay single eggs at a time. Eggs will be covered with a mixture of secretions and wood chips by female adults. Larvae (1st–2nd instar) feed on the phloem, while 3rd–5th instar can bore into the phloem and the cambium. Massive tunnels, including three shapes (inverted “L”, inverted “T”, and inverted “Z”), were observed in the trunk of each tree, and resulted in swelling of the outer bark. P. tsushimanus has one life cycle per year in Shanghai. Both adults and larvae (3rd–5th instar) overwinter from early November to early April. Adults overwinter in grooves on the underside of branches or at branch nodes, and larvae overwinter in tunnels. Five larval instars of P. tsushimanus were determined according to Dyar's and Crosby's rules. The biological traits and life history of P. tsushimanus have been identified and can provide guidance in terms of pest control and plantation management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camphor tree, Cinnamomum camphora, is an indigenous tree species of South China, Korea, Vietnam, and southern Japan (Li et al. 1982; Zhang et al. 2019). It is cultivated in numerous countries as an ornamental or as a medicinal plant for muscular strains, inflammation, and rheumatic treatment (Babu et al. 2003). In China, C. camphora is widely cultivated in the tropical and subtropical regions as an afforestation species in mountainous areas, and as a common and important landscape species in many urban areas in south China, especially in Shanghai City (Fan 2018; Zhang et al. 2018). In addition, this species is a good resource for furniture manufacturing and for camphor and essential oil extraction (Guo et al. 2016). The essential oil has strong bioactivity against insect pests when applied as a fumigant (Nenaah and Ibrahim 2011), a contact agent (Guo et al. 2016), and as a repellent (Nenaah and Ibrahim 2011). However, C. camphora can be infected by some insect pests, such as Pagiophloeus tsushimanus (Chen et al. 2020), Orthaga achatina Butler (Lepidoptera: Pyralidae) (Yan et al. 2018), and Orthaga olivacea Warre (Wei et al. 2008).
P. tsushimanus was originally described by Morimoto (1982) and collected from Tsushima Island of Kyushu, Japan. Since the initial report of P. tsushimanus in China in 2014, it has become a destructive insect pest of C. camphora plantations in Shanghai (Huang et al. 2014), and is a potential threat in eastern urbanized areas (Gu et al. 2017; Chen et al. 2020). Therefore, an effective pest management strategy based on the biological traits of P. tsushimanus is extremely urgent.
A good understanding of the biological traits of this pest is fundamental for developing effective pest control strategies (Reznik et al. 2015; Qin et al. 2016; Shahid et al. 2017). To date, limited studies have been reported on P. tsushimanus, such as pest identification (Huang et al. 2014; Zhang et al. 2018), mating and oviposition behaviour (Zhang et al. 2017), development (Li et al. 2019), and stability of reference genes (Chen et al. 2020). A lack of information on the biological traits and life history has limited research on management strategies against this pest. In this study, we aimed to fill the gap by investigating the damage and life history, and by determining the larval instar of P. tsushimanus. These results will be valuable for both practical application and further studies.
Materials and methods
Study area
The research was carried out in Maogang, Songjiang District, Shanghai (30°56′6.15″N, 121°12′32.76″E). Annual mean temperature is 17 °C; there is approximately 125 rainy days per year, with an annual rainfall of 1157 mm; the annual sunlight is 1909.2 h. A 200-hectare plantation cultivated with 15-year-old C. camphora was selected where an outbreak of P. tsushimanus infestation was first discovered in 2014 (Huang et al. 2014). Mean height (HT) of the trees (n = 60) was 5.0 ± 1.2 m (mean ± standard error (SE)), and the diameter at breast height (DBH) was 21.1 ± 6.3 cm (n = 60, mean ± SE) (Table S1). The distance between trees was 3.0 m. A few other insects have occasionally infested C. camphora in this plantation such as Orthaga olivacea Warren, Thalassodes quadraria Guenée, Zeuzera coffeae Neitner, and Diaphania perspectalis Walker.
Damage and life history
To study the occurrence and duration of the developmental stages of P. tsushimanus in the field, six 20 m × 20 m sample plots were established. Ten trees that had been infested by P. tsushimanus were randomly selected, sampled in each plot and marked with red paint. From March 2016 to November 2018, surveys were carried out every ten days to collect insect samples at different stages of development. C. camphora plantations in other administrative districts of Shanghai were evaluated by random sampling.
Feeding, mating, and oviposition of adults after emergence were observed and recorded. Adult damage was investigated in the lower tree crowns in east, west, south, and north directions. Three fresh tunnels were dissected on each tree which were located by a cue of frass or powdery refuse accumulation at the entrance hole in the trunks (Cui et al. 2019). The trunks were peeled to reveal the complete structure of tunnels, and the development and associated damage of all immature larvae were recorded.
P. tsushimanus preparation before overwintering was observed; its distribution within the trunk was investigated to determine which stages overwintered, and the performance of the stages was recorded simultaneously. The surveyed adults and larvae were brought to the laboratory of Nanjing Forestry University and theadults were bred using fresh C. camphora twigs in plastic containers. Larvae were bred using an artificial diet of twig powder in a petri dish. The life cycle of P. tsushimanus was determined by both field investigations and laboratory breeding.
Larval instar determination and data analysis
Larvae (n = 461) were collected during field investigations and taken to the insect laboratory of Nanjing Forestry University. The highly ossified head capsules of the larvae were removed, cleaned, and fixed on a strip of adhesive tape (Gao et al. 2017). The head capsule width (HCW) and head capsule length (HCL) of each larva were measured under a Zeiss Discovery V20 stereomicroscope (Fig. 1).
According to their frequency distributions, 100-µm groups of HCWs and HCLs were established and normal multimodal fitting performed, each peak represented an instar (Panzavolta 2007). The intersection point of two adjacent normal curves was regarded as the point dividing larval instars (Panzavolta 2007). The number of larval instars obtained from the frequency distribution analysis was verified according to Dyar's and Crosby's (Brooks') rules (Dyar 1890; Crosby 1973; Loerch and Cameron 1983). A coefficient of variation less than 20% and a Crosby's index less than 10% were used as criteria (Loerch and Cameron 1983; Dallara et al. 2012). Mean values, coefficients of variation, Brooks' index (Eq. 1), and Crosby's index (Eq. 2) were calculated by Microsoft Office Excel 2016 edition (Microsoft Corporation, Redmond, WA, USA). Pearson correlation analysis and linear regression analysis of the two morphological measurement data sets were performed via SPSS version 20.0 edition (IBM, GeoHack, NY, USA) (Qian and Song 2013). All figures were plotted with Origin version 8.5 edition (Origin Lab, Northampton, MA, USA).
where Xn is the mean of each measurement index for larvae (n instar), Xn − 1 is the mean of each measurement index for larvae (n − 1 instar), bn denotes Brooks' index of larvae (n instar), and bn − 1 denotes Brooks' index of larvae (n − 1 instar).
Results
Infestation traits
According to field investigations, P. tsushimanus is a monophagous weevil pest and C. camphora is the unique host tree species. Areas infested with P. tsushimanus have increased yearly, and C. camphora plantations in all administrative districts of Shanghai have been seriously damaged by P. tsushimanus.
Both adults and larvae of P. tsushimanus can damage host trees and have seriously reduced the economic and aesthetic value of C. camphora. In particular, P. tsushimanus overwintered with adults and larvae, the adults in grooves on the underside of branches or branch nodes, the larvae in tunnels.
Adult damage
After adults emerge from the trunks of host trees, they climb up to the crown and feed on the tender bark of twigs (red arrows, Fig. 2a). Aggregation of adults were often observed during field investigations and indoor feeding (Fig. S1) with the most active period occurring from July to September. C. camphora twigs damaged by P. tsushimanus adults shrink and crack (Fig. 2b). Occasionally, adults feed on newly emerged buds and the buds shrink. The growth of host trees is seriously affected after severe damage by a large number of adults.
During feeding intervals, adults have the habit of putting their heads downwards near the node of the branch. Adults spread by crawling frequently, not flying, and thanatosis is common. When frightened by the noise around or attacked by other animals, adults immediately fell from the shoots to the ground. The colour and granular bulge on the adult integument are similar to the bark of C. camphora, indicating mimesis and camouflage (red arrow, Fig. 2c).
Mating frequently occurs on sunny sides of the crown. In this study, laboratory observations found that adults mate and oviposit repeatedly. When adults copulate, the male mounts the dorsum of the female, clasping with forelegs, controlling the female's elytra with the middle legs and clenching the end of the abdomen with the hind legs (Fig. S2).
After mating, females crawl along the trunk below the crown to find a suitable oviposition site. An egg chamber is created by the female with its rostrum and mouthparts into the cambium (Fig. 2d). The abdomen is bent downwards, the ovipositor extended and the egg laid. All females lay single eggs at a time, with one in each chamber. Once laid, the egg is covered with a mixture of secretions and wood chips ( red arrows, Fig. 2e). Before overwintering, a groove which is slightly larger than the adult body is made on the underside of the branch or branch node (Fig. 2f).
Larva damage
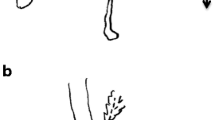
Larvae (1st–2nd instar) feed on the phloem and produce small, fine frass. Following this, 3rd–5th instar can bore into both phloem and cambium and produce large amounts of thick frass. After larval excavation, three shapes of tunnels (inverted “L”, inverted “T”, and inverted “Z”) were recorded. Newly hatched larvae initially bore in a horizontal direction but when they develop into the last instar, they bore a perpendicular gallery to form a pupal chamber which is vertical to the horizontal galleries and finally form an inverted L-shaped tunnel (Fig. 3a). Some last-instar larvae created perpendicular galleries from the middle of the horizontal tunnel to finally form an inverted T-shaped tunnel (Fig. 3b). Sometimes the newly hatched larvae may bore in a vertical direction but feed in a horizontal direction. However, other last-instar larvae bore perpendicularly to the end of the horizontal tunnel and to eventually form a pupal chamber. These three sections formed an inverted Z-shaped tunnel (Fig. 3c).
Frass is pushed continuously by larvae so that frass near the hole of tunnel is expelled first and is scattered on the trunk surface (red arrow, Fig. 3d). The colour of a fresh tunnel is usually brown and darkened when the larva pupated within it. Larva at the pre-pupal stage stopped feeding and retreated to a vertical tunnel for pupation. During this period, a large amount of flocculent wood chips is made by the larva and piled beneath the larval head (Fig. 3b). Massive tunnels were observed in the trunk of each tree (red arrows, Fig. 3e) and resulted in swelling of the outer bark (red arrows, Fig. 3f), thus seriously affecting the growth of the tree, and sometimes resulting in death.
Life history
The life cycle of P. tsushimanus consists of four stages – egg, larva, pupa, and adult (Fig. 4a). According to field investigations, P. tsushimanus has one life cycle per year in Shanghai (Figs. 4b, 5). Both adults and larvae (3rd–5th instar) overwinter from early November to the following early April. Overwintering with eggs, larvae (1st–2nd instar) or pupae has not been found in the field.
Overwintering adults start activity in mid-April, crawling upwards to twigs for feeding. Mating is initiated in late April, with the oviposition period lasting from late April to early June. Eggs begin hatching in early June, and larvae start to pupate in late July and end in early September. Emergence of adults starts from early September to late September (Fig. 5).
Overwintering larval feeding is initiated in mid-April and ends in early July. Larvae pupate from mid-May to early July. Adults emerge from mid-June to early August, with mating and oviposition from mid-July to late September. The eggs begin hatching in late August (Fig. 5).
Larval instar determination
The HCWs of P. tsushimanus larvae range from 480 to 3,100 μm (n = 461), and HCLs from 620 to 4,130 μm (n = 461) (Table S2). There are normally five peaks in the frequency distributions of both HCWs (Fig. 6a) and HCLs (Fig. 6b). Each peak represents an instar, and the intersection points of four normal distribution curves reveal four instar cut-off points. Therefore, these peaks indicate five larval instars of P. tsushimanus.
Crosby's indexes for the mean values of HCWs and HCLs measured for different instars were all less than 0.1, and the coefficients of variation of the two morphological measurement data for the different instars were all less than 15%, which suggests the determination of five larval instars (Table 1).
The natural logarithmic values of the mean HCW (r = 0.985; n = 5) and HCL (r = 0.990; n = 5) are positively correlated with instar. The linear regression between the natural logarithmic values of mean HCW and instars was highly significant (R2 = 0.970; F = 96.044; df = 1, 3; P = 0.002) (Fig. 7a). The same linear regression was observed between the natural logarithmic values of mean HCL and instars (R2 = 0.981; F = 153.527; df = 1, 3; P = 0.001) (Fig. 7b). These results strongly support the determination of larval instars.
In addition, the mean HCW was positively correlated with the mean HCL (r = 0.983, n = 461), and regression between HCW and HCL was highly significant (R2 = 0.966; F = 12,976.129; df = 1, 459; P < 0.0001) (Fig. 8). Consequently, it is concluded that the number of larval instars of P. tsushimanus is five. The results suggest that either HCL or HCW could be a reliable index for larval instar determination.
Discussion
The results of the life history and larval instar of P. tsushimanus indicate that the weevil produces one generation per year in Shanghai and overwinters with adults and larvae (3rd–5th instar). Other wood borer weevils such as Eucryptorrhynchus chinensis Oliver (McAvoy et al. 2014) and E. brandti Harold (Luo et al. 2016) also have similar life cycles and overwinter with larvae and adults (Yang et al. 2008; Yu et al. 2012). Overwintering with late-instar larvae and adults may be because eggs, young larvae, and pupae have less resistance to cold temperatures. C. camphora is an evergreen tree species, and branches and leaves naturally provide shelter for overwintering adults. However, the mechanism of staggered different stages during overwintering remains unclear. Overwintering adults mainly emerge in mid-April which is in line with the active growth period of host trees. Therefore, new buds or young twigs of C. camphora are damaged by newly emerged adults, which negatively affects tree growth.
Larval boring in trunks can cut off vascular tissues and hinder the transport of water and nutrients. A heavy infestation of multiple larvae of P. tsushimanus will result in swelling of the outer bark or in the death of host trees. In addition, we observed that many flocculent wood chips, produced by last-instar larvae excavating, accumulated beneath the larval head in pupal chambers. These chips may prevent rainwater or natural enemies from entering, which should be verified in future studies. Although P. tsushimanus is currently identified as a specialist pest that infests only C. camphora in the field, a laboratory study showed that its larvae can complete their life cycle by feeding on an artificial diet of powdered twigs of Cinnamomum chekiangensis Nakai and Phoebe chekiangensis Shang (Laurales: Lauraceae). In addition, the developmental duration of larvae feeding on this artificial diet was significantly longer than those feeding on twigs of C. camphora (Li et al. 2019). C. chekiangensis and C. camphora are in the same genus, while P. chekiangensis does not, however, the three species belong to the same family. Specific nutritional acquisition from C. camphora or detoxifying enzyme activities of P. tsushimanus may be the reasons for its host specificity (Li et al. 2019). Assays on the growth and development of P. tsushimanus feeding on various species should be studied in the future, for a comprehensive evaluation of the risk of shifts in hosts.
Instar determination from field-collected larvae is a common problem in insect pest monitoring. Commonly, the morphological measurement data of different instars overlap to some extent and make it difficult to determine the accurate instar of a sample larva (Yazdani et al. 2015). Various techniques have been applied to estimate the overlap between adjacent instars in order to provide classification rules for field-collected larvae (Fox et al. 1972; Frampton 1986; Mcclellan and Logan 1994; Goldson et al. 2001; Hammack et al. 2003). Head capsule width is the most frequent measure used to determine larval instar and has been widely applied to other weevils, such as Eurhinus magnificus Gyllenhal (Coleoptera: Curculionidae), Cosmopolites sordidus Germar (Coleoptera: Curculionidae), and Sitophilus zeamais Motchulsky (Coleoptera: Curculionidae) (Logan et al. 1998; Merville et al. 2014). The method of frequency distributions of head capsule width (HCW) and head capsule length (HCL) has been widely applied in larval instar determination such as Lobesia botrana (Denis and Schiffermüller) (Lepidoptera: Tortricidae) and Rhyssomatus subtilis Fiedler (Coleoptera: Curculionidae) (Delbac et al. 2010; Cazado et al. 2014). There were five normal peaks in the frequency distributions of HCWs and HCLs of P. tsushimanus according to this method which followed the general standard that morphological measurement data of individuals at the same developmental stage are distributed normally (Hunt and Chapman 2001).
The results of larval instar determination were generally verified by the application of Dyar's and Crosby's (Brooks') rules (Dyar 1890; Crosby 1973; Loerch and Cameron 1983). Thus, we used this method to verify larval instar determination in P. tsushimanus. The increase in HCW of each larval instar was consistent with Dyar's rule. If the logarithmic head capsule measurements correspond to the number of larval instars, the perfect geometrical progression of the HCW is represented by a straight line (Gaines and Campbell 1935). Both HCWs and HCLs of P. tsushimanus corresponded to each larval instar and exhibited a straight line, which supported five instars of P. tsushimanus larvae. In addition, there is a highly significant linear relationship between HCW and HCL which confirms our conclusion convincingly.
To date, no effective strategies have been developed to control P. tsushimanus. The previous method to control larvae was injecting conventional insecticides into tree trunks (Fan 2018). However, the effect was poor because of the incorrect period of control. The results of our study suggest that the essential period is from July to September because larvae and adults are active during this period. Also, most P. tsushimanus larvae are in the first instar which is highly sensitive to insecticides. The control of adults by prompting thanatosis or apparent death to reduce density could be performed soon after emergence when most adults disperse to the tree canopy for maturation feeding and mating.
The knowledge of biological traits of P. tsushimanus will provide guidance for managing this pest. However, there is still much to be studied such as its physiology (e.g., resistance to plant secondary metabolites), chemical ecology (e.g., attractants), and molecular biology (e.g., functional genes). Furthermore, investigations of natural enemies and development of semiochemical lures may be effective measures of control. Sex pheromones or host tree volatiles are used by many insects for mating or host location (Lee et al. 2005; Chung et al. 2013; Liu and Zhou 2016). The behavioural traits of P. tsushimanus adults suggested that aggregation pheromones of adults are produced by males (Zhang et al. 2017). Secretions produced by female adults that cover eggs might contain a host-marking or oviposition pheromone to deter other females from laying eggs (Rosi et al. 2001; Zhang et al. 2017). In addition, P. tsushimanus only infested C. camphora in the field, which implies that host tree volatiles are a potential attractants. Meanwhile, identification and application of sex pheromone attractants of P. tsushimanus could be conducive to the development of an alternative, eco-friendly method for controlling this pest.
Conclusions
P. tsushimanus is a monophagous weevil pest, feeding only on C. camphora. C. camphora plantations in all administrative districts of Shanghai have been seriously infected. Adults often aggregate for feeding on the tender bark of twigs, and occasionally on newly emerged buds. After damage, twigs will shrink and crack and buds will shrink. Adults tend to repeatedly mate and oviposit, and all females lay single eggs at a time. All eggs are covered with a mixture of secretions and wood chips by female adults.
P. tsushimanus has one life cycle per year in Shanghai. Both adults and larvae (3rd–5th instar) overwinter from early November to the following early April. Adults overwinter in grooves on the underside of branches or branch nodes and larvae in tunnels. Five larval instars of P. tsushimanus were identified.
The biological traits and life history of P. tsushimanus have been shown and can provide guidance in pest control and plantation management.
References
Babu KN, Sajina A, Minoo D, John CZ, Mini PM, Tushar KV, Rema J, Ravindran PN (2003) Micropropagation of camphor tree (Cinnamomum camphora). Plant Cell Tissue Organ Cult 74(2):179–183
Cazado LE, Van Nieuwenhove GA, Brien CWO', Gastaminza GA, Murúa MG (2014) Determination of number of instars ofRhyssomatus subtilis(Coleoptera: Curculionidae) based on head capsule widths. Fla Entomol 97(2):639–643
Chen C, Li SL, Zhu H, Fan BQ, Wang Y, Hao DJ (2020) Identification and evaluation of reference genes for gene expression analysis in the weevil pest Pagiophloeus tsushimanus using RT-qPCR. J Asia-Pac Entomol 23(2020):336–344
Chung AYC, Maycock CR, Khoo E, Hastie A, Kimjus K (2013) New records of insects associated with Bornean endemic dipterocarp seedlings. J Trop For Sci 25(1):5–11
Crosby TK (1973) Dyar's rule predated by Brooks' rule. N Z Entomol 5(2):175–176
Cui ZJ, Zhang YL, Zhang X, Luo ZH, Zhang P, Golec J, Poland TM, Zalucki MP, Han P, Lu ZZ (2019) Life history and mortality factors of Agrilus mali Matsumura (Coleoptera: Buprestidae) in wild apples in Northwestern China. Agric For Entomol 21:309–317
Dallara PL, Flint ML, Seybold SJ (2012) An analysis of the larval instars of the walnut twig beetle, Pityophthorus juglandis Blackman (Coleoptera: Scolytidae), in northern California black walnut, Juglans hindsii, and a new host record for Hylocurus hirtellus. Pan-Pacific Entomol 88(2):248–266
Delbac L, Lecharpentier P, Thiery D (2010) Larval instars determination for the European grapevine moth (Lepidoptera: Tortricidae) based on the frequency distribution of head-capsule widths. Crop Prot 29(6):623–630
Dyar HG (1890) The number of molts in lepidopterous larvae. Psyche 5:420–422
Fan BQ (2018) Control efficacy of using trunk injection method against the larva of Pagiophloeus tsushimanus. For Pest Dis 37(3):50–52 (In Chinese)
Fox RC, Anderson NH, Garner SC, Walker AI (1972) Larval head capsules of the nantucket pine tip moth. Ann Entomol Soc Am 65(2):513–514
Frampton ER (1986) Determination of the number of larval instars of Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) using probit analysis. N Z J Zoolo 13:107–111
Gaines JC, Campbell FL (1935) Dyar's rule as related to the number of instars of the corn ear worm, Heliothis obsoleta (Fab.), collected in the field. Ann Entomol 28:445–461
Gao CQ, RÉDEI D, Shi XQ, Cai B, Ling K, Gao S, Bu WJ (2017) A comparative study of the abdominal trichobothria of Trichophora, with emphasis on Lygaeoidea(Hemiptera: Heteroptera). Eur J Entomol 114:587–602
Goldson SL, McNeill MR, Proffitt JR, Baird DB (2001) Seasonal variation in larval-instar head-capsule sizes of Argentine stem weevil, Listronotus bonariensis (Kuschel) (Coleoptera: Curculionidae). Aust J Entomol 40:371–375
Gu TZ, Zhang CC, Su P, Fan BQ, Hao WY, DJ, (2017) Ultrastructure of antennal sensilla of Pagiophloeus tsushimanus Morimoto (Coleoptera: Curculionidae) with scanning electron microscope. J Nanjing For Univ (Nat Sci Edit) 41(4):1–6 (In Chinese)
Guo SS, Geng ZF, Zhang WJ, Liang JY, Wang CF, Deng ZW, Du SS (2016) The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int J Mol Sci 17(11):1836
Hammack L, Ellsbury MM, Roehrdanz RL, Pikul JL Jr (2003) Larval sampling and instar determination in field populations of northern and western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 96(4):1153–1159
Huang JH, Wu SY, Gao L, Yang LJ (2014) Diagnosis and damage of weevil pest Pagiophloeus tsushimanus on camphor tree. J Zhejiang A & F Univ 31(5):764–767 (In Chinese)
Hunt G, Chapman RE (2001) Evaluating hypotheses of instar-grouping in arthropods: a maximum likelihood approach. Paleobiology 27(3):466–484
Lee S, Lee DW, Boo KS (2005) Sex Pheromone Composition of the Diamondback Moth, Plutella xylostella (L.) in Korea. J Asia-Pac Entomol 8(3):243–248
Li XW, Li J, Werff H (1982) Flora reipublicae popularis sinicae. Science press, Beijing, China (In Chinese)
Li SY, Chen C, Li H, Fan BQ, Wang Y, Hao DJ (2019) Effects of feeding on diets containing components of different plants on the development and detoxifying enzyme activities in Pagiophloeus tsushimanus (Coleptera: Curculionidae) larvae. Acta Entomol Sin 62(11):1286–1296 (In Chinese)
Liu L, Zhou Q (2016) Olfactory response of female Bactrocera minax to chemical components of the preference host citrus volatile oils. J Asia-Pac Entomol 19(3):637–642
Loerch CR, Cameron EA (1983) Determination of larval instars of the bronze birch borer, Agrilus anxius (Coleoptera: Buprestidae). Ann Entomol Soc Am 76(6):948–952
Logan JA, Bentz BJ, Vandygriff JC, Turner DL (1998) General program for determining instar distributions from head capsule widths: example analysis of mountain pine beetle (Coleoptera: Scolytide) data. Environ Entomol 27(3):555–563
Luo W, Ji YC, Wen JB (2016) Application of a frequency distribution method for determining instars of Eucryptorrhynchus brandti (Coleoptera: Curculionidae) from several morphological variables. Biocontrol Sci Technol 26(10):1–12
Mcavoy TJ, Salom SM, Yu B, Ji HL, Du YZ, Johnson N, Reardon R, Kok LT (2014) Occurrence and development of Eucryptorrhynchus brandti and E. chinensis (Coleoptera: Curculionidae) on Ailanthusaltissima trees subjected to different levels of mechanical damage. Biocontrol Sci Technol 24(1):65–79
Mcclellan QC, Logan JA (1994) Instar determination for the gypsy moth (Lepidoptera: Lymantriidae) based on the frequency distribution of head capsule widths. Environ Entomol 23(2):248–253
Merville A, Vallier A, Venner S, Siberchicot A, Bel-Venne MC (2014) Determining the instar of a weevil larva (Coleoptera: Curculionidae) using a parsimonious method. Eur J Entomol 111(4):567–573
Morimoto K (1982) The family Curculionidae of Japan. I subfamily Hylobxinae Esakia 19:51–121
Nenaah GE, Ibrahim SIA (2011) Chemical composition and the insecticidal activity of certain plants applied as powders and essential oils against two stored-products coleopteran beetles. J Pest Sci 84(3):393–402
Panzavolta T (2007) Instar determination for Pissodes castaneus (Coleoptera: Curculionidae) using head capsule widths and lengths. Environ Entomol 36(5):1054–1058
Qian H, Song JS (2013) Latitudinal gradients of associations between beta and gamma diversity of trees in forest communities in the new world. J Plant Ecol 6(1):12–18
Qin QJ, Liu S, Li S, Zhang W, He YZ (2016) Role of photoperiod in the development and reproduction of Harmonia axyridis (Coleoptera: Coccinellidae). Biocontrol Sci Technol 26(1):116–124
Reznik SY, Dolgovskaya MY, Ovchinnikov AN, Belyakova NA (2015) Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J Appl Entomol 139(4):241–249
Rosi MC, Isidoro N, Colazza S, Bin F (2001) Source of the host marking pheromone in the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). J Insect Physiol 47(9):989–995
Shahid AA, Mudasir AD, Mahendiran G, Aijaz AW (2017) The first record of pear psylla Cacopsylla bidens (Hemiptera: Psyllidae) from India along with notes on seasonal occurrence and some elements of its biology. Orient Insects 52(1):101–111
Wei SJ, Xu FL, Hua FL, Lu JD, Zhang CG, Chen XX (2008) A camphor insect pest-bionomics of Orthaga olivacea. Chin Bull Entomol 45(4):562–565 (In Chinese)
Yan Q, Li HD, Chen Y, Ye ZF, You XY, Zhou J, Mu LF, Liu SJ, Kong XB, Khuhro SA, Dong SL (2018) Identification and field evaluation of the sex pheromone of Orthaga achatina (Lepidoptera: Pyralidae). J Chem Ecol 44:886–893
Yang GJ, Yong HL, Wang XP (2008) The biological characters and behavior of Eucryptorrhynchus chinensis. Chin Bull Entomol 45(1):65–69 (In Chinese)
Yazdani M, Feng Y, Glatz R, Keller MA (2015) Host stage preference of Dolichogenidea tasmanica (Cameron, 1912) (Hymenoptera: Braconidae), a parasitoid of Epiphyas postvittana (Walker, 1863) (Lepidoptera: Tortricidae). Austral Entomol 54:325–331
Yu QQ, Chen C, Liu ZK, Sun YW, Cao CJ, Bao S, Wen JB (2012) Occurrence and life-history of Eucryptorrhynchus chinensis in Lingwu. Ningxia J Appl Entomol 49(4):1005–1009 (In Chinese)
Zhang CC, Wamg M, Su P, Fan BQ, Wang Y, Hao DJ (2017) Mating and oviposition behaviors of Pagiophloeus tsushimanus (Coleoptera: Curculionidae). Annu Rev Entomol 42(10):207–230 (In Chinese)
Zhang CC, Gu TZ, Su P, Fan BQ, Wang Y, Hao DJ (2018) Identification and phylogenetic position of Pagiophloeus tsushimanus based on COI and rDNA sequences. For Res 31(3):78–87 (In Chinese)
Zhang Y, Zhang DJ, Li X, Zhang J (2019) Contribution of soil fauna to the degradation of recalcitrant components in Cinnamomum camphora foliar litter in different-sized gaps in Pinus massoniana plantations. J For Res 30(3):931–941
Acknowledgements
We thank Yuefeng Zhang, Liang Chen, and Yanbo Sun (Forest Station of Shanghai) for their technical assistance. We also thank Tian Xu (State University of New York), Ruixu Chen (Nanjing Forestry University), You Li (University of Florida), Cindy Cheng (University of British Columbia), and Yongyu Huang (Leibniz Institute of Plant Genetics and Crop Plant Research) for thoroughly checking the language.
Author information
Authors and Affiliations
Contributions
CC and DH conceived and designed the experiments and wrote the manuscript. CC, CZ, SL, and HZ performed the experiments and analysed the data. BF, YW, PS, and YH collected the insects. All authors reviewed and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding editor: Yu Lei
The online version is available at http://www.springerlink.com.
Project funding: This study was financially supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1077), the Science and Technology Commission of Shanghai Municipality (18,391,903,200), and the Shanghai Landscaping & City Appearance Administrative Bureau (G161206).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, C., Zhang, C., Li, S. et al. Biological traits and life history of Pagiophloeus tsushimanus (Coleoptera: Curculionidae), a weevil pest on camphor trees in China. J. For. Res. 32, 1979–1988 (2021). https://doi.org/10.1007/s11676-020-01227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01227-2