Abstract
Castanopsis fargesii is a good afforestation plants and various microorganisms play important roles in mediating the growth and ecological functions of this species. In this study, we evaluated changes in microbial communities in soil samples from C. fargesii forests. The phospholipid fatty acid (PLFA) biomarker method was used to obtain bacteria, fungi, actinomycetes, gram-positive bacteria (G+), gram-negative bacteria (G−), aerobic bacteria, and anaerobic bacteria to investigate spatiotemporal changes in microbial communities during the growing season. The results show that soil microorganisms were mainly concentrated in the upper 20-cm layer, demonstrating an obvious surface aggregation (P < 0.05). Large amounts of litter and heavy rainfall during the early growing season resulted in the highest PLFA contents for various microorganisms, whereas relatively low and stable levels were observed during other times. The dominant species during each period were bacteria. G+ or aerobic bacteria were the main bacterial populations, providing insights into the overall trends of soil bacterial PLFA contents. Due to the relative accumulation of refractory substances during the later stages of litter decomposition, the effects of fungi increased significantly. Overall, our findings demonstrate that the main factors influencing microbial communities were litter, rainfall, and soil field capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil microorganisms are important components and active participants in material circulation, energy flow, and information transmission in forest ecosystems (Zhang et al. 2017). Because of the sensitivity of these organisms to the living microenvironment, microbial community structure can be used as a sensitive index to evaluate changes in soil ecosystems. Studying the community structure and diversity of soil microbes can provide important insights into the stability of soil ecosystems, the buffering capacity of the soil against ecological deterioration, and soil quality (McGuire et al. 2010; Bardgett and van der Putten 2014; Huang et al. 2015). Additionally, the ecological functions of microbes and the protection and utilization of forest resources are important areas of study. Several studies have been carried out on forest soil microbial communities with a focus on the effects of seasonal changes (Stevenson et al. 2014; Li et al. 2017), vegetation types (Thoms et al. 2010; Yao et al. 2018), altitude (Shen et al. 2013), precipitation (Chen et al. 2015a, b; Nielsen and Ball 2015), and nitrogen addition (Contosta et al. 2015; Zhou et al. 2017b).
Castanopsis fargesii is a dominant species in evergreen broad-leaved forests in mid-subtropical regions. The species has a wide distribution, and high economic, ecological, and scientific value. To date, studies of C. fargesii forests have primarily focused on leaf phenolic compounds (Huang et al. 2017), ecological footprints of population differentiation (Li et al. 2014), soil organic carbon (Gong et al. 2015), and ectotrophic mycorrhiza (Wang et al. 2011). No studies have examined soil microorganisms associated with C. fargesii forests.
In this study, the soil of C. fargesii forests was evaluated using the phospholipid fatty acid (PLFA) method to explore vertical, temporal, and community structural changes in bacteria, fungi, and actinomycetes in different growing seasons. Redundancy Analysis (RDA) was used to explore the main factors that might affect changes in soil microbial communities in order to provide a basis for further evaluation of the stability of C. fargesii forests and the study of soil microorganisms.
Materials and methods
Site description
The study area was located in the Dagangshan Mountain Forest Eco-system Research Station in Fenyi County, Jiangxi Province (114°30′–114°45′E, 27°30′–27°50′N). This region has a humid subtropical monsoon climate, radiation of 486.6 kJ/cm2, an average annual temperature of 15.8 °C, and an average annual precipitation of 1590.9 mm. Precipitation between April and June accounts for approximately 44.6% of the total annual rainfall. The soil is an ultisol, a highly weathered, leached soil of humid areas and formed from kaolinite, a layered clay. The parent rocks are granite, sandstone, phyllite, and plate shale. The soil is acidic with a sandy loam texture.
The C. fargesii forest was 50 years old, with an average height of 23 m, an average diameter at breast height of 25 cm, a smaller change in forest type, and a canopy density of 80%. Understory vegetation included Syzygium buxifolium Hook et Arn., Viburnum dilatatum Thunb., Cinnamomum porrectum (Roxb.) Kosterm, Machilus velutina Champ, Itea chinensis Hook et Arn., and Lophatherum gracile Brongn.
Sample collection
Five sample plots 20 m × 20 m were randomly selected, each with five sampling points in an “S” shape. Sampling was centred on each point. During April (early growing season: flowering period), August (growing season: flourishing period), October (growing season: fruit maturity period), and December (dormant season), earth drills were used to collect the centre points of different layers of soil (0–20, 20–40, 40–60, and 60–100 cm) at 0.5 m. These samples were mixed with five samples of the same soil layer to obtain a mixed soil sample (Fig. 1). Half the sample was kept at 4 °C and used to determine PLFA (Bardgett et al. 1996), and the other half was- air dried, processed through 2- and 0.149-mm sieves, and used to determine organic carbon contents, pH, particle compositions, and physical and chemical properties.
Measurement methods
Determination of PLFA
The biomarker method was used for the analysis of PLFA (Kourtev et al. 2002). The samples were dissolved in 200 μL n-hexane and a 19:0 methyl ester used as the internal standard to produce a methylated fatty acid sample. A MIDI software system-based gas chromatograph (Hewlett-Packard 6890; Agilent, USA) was used for determination of PLFA quality (nmol g−1). The peak time and area of fatty acid methyl ester were used for analysis of the quality and distribution of microbial communities (Zhang et al. 2013a). The absolute amount of PLFA was calculated by the area normalization method using the following formula:
Soil rainfall and temperature
Rainfall is based on data from weather stations in Fenyi County; soil temperatures were measured by burying a U22-001 automatic thermometer at each sampling point (Table 1).
Determination of litter
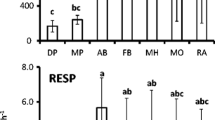
A 1 m × 1 m nylon net litter collector (1 m from the ground) was placed at each of the four corners and the centre of each plot to collect litter at different periods (Fig. 2).
Determination of soil physicochemical properties (State Forestry Bureau 1999) (Table 1)
Measurement of water holding capacity was carried out using the ring knife method. Determination of soil organic carbon content was performed using the K2Cr2O7 oxidation external heating method, and determination of pH was carried out by the potentiometric method (water to soil ratio of 2.5:1). The straw method was used to measure soil particle composition.
Statistical analysis
Soil microbial markers
Microorganisms are characterized by specific PLFA; therefore, based on previous studies, soil microbial markers were established (Table 2) (Zelles 1999; Bossio et al. 2006; Huygens et al. 2011; Breulmann et al. 2012; Moche et al. 2015; Wang et al. 2015; Moon et al. 2016; Richter et al. 2018).
Data analysis
One-way analysis of variance and Duncan tests were performed with Excel 2010 and SPSS 20.0 software (SPSS Inc., Armonk, NY, USA), and redundancy analysis (RDA) was performed using Canoco 4.5 software.
Results and analysis
Spatiotemporal changes in soil bacteria, fungi, and actinomycetes
The PLFA contents of bacteria and actinomycetes during each period ranged from 3.8 to 12.6 nmol g−1 and from 0.4 to 1.9 nmol g−1, respectively, accounting for 57.1–68.2% and 46.4–54.9% of the vertical profile, respectively, and showed strong surface aggregation. There were no significant changes in the other layers. During April and August, the fungal PLFA content in the upper 20-cm soil layer was relatively high, accounting for 51.2–57.6% of the vertical profile. Surface aggregation was obvious, and vertical changes in the profile during October and December showed gradual changes. The differences in changes between layers were not significant (Fig. 3).
At depths of < 60 cm, the PLFA contents in bacteria and actinomycetes during different periods were similar, with the highest values in April; there were no significant differences in August, October, and December. The PLFA level in soil fungi at a depth of < 20 cm was the same as that of bacteria and actinomycetes, whereas the PLFA contents in fungi at 20–40 and 40–60 cm depths varied. In addition, the PLFA contents of bacteria, fungi, and actinomycetes did not change significantly during the different periods after depths of more than 60 cm.
Soil F/B is an evaluation index for the dominance of bacteria and fungi (Fang et al. 2000). The values tended to increase as depths increased during each period. In April, there was a strong change between layers with significant differences between < 20, 20–60, and 60–100 cm depths. In contrast, in August, October, and December, changes were consistent between the layers, with significant differences between < 40 and 40–100 cm (Table 3). A comparison of soil layers during different periods showed that the lowest F/B value was only 0.1–0.3 in April. In August, the F/B value in the same layer increased significantly, reaching 0.3–0.5. There were no significant changes between October and August in the same layer; however, after 12 months, the F/B value for the < 40 cm layer re-appeared (Table 3).
Spatiotemporal changes in soil G+ and G− bacteria
April was the most active period for G+ bacteria in the year, and G+ bacterial contents at depths of < 60 cm were significantly higher than those in August. However, there were no significant changes in August, October, and December. The content of G+ bacteria in the < 20 cm soil layer was significantly higher than in the lower layers at different times, and there was a clear surface aggregation occurrence. Additionally, variations in bacterial profiles differed for the 20–100 cm profile (Fig. 4).
Spatiotemporal changes in PLFA contents of G− bacteria were more complex. The PLFA levels in the < 20 cm layer were dramatically altered during the four periods, and changes at depths of 20–40 cm and 40–60 cm were substantial from April to August. From October to December, the levels were relatively stable, and there were no significant changes at depths of 60–100 cm. In April and August, the vertical variations in G− bacterial PLFA contents were not obvious; however, in October and December, a significant decrease was observed as soil depth increased.
The G+/G− ratios were 6.2–38.5, indicating a wide range of changes. There was no obvious regularity in the characteristics of changes in time and vertical profiles, and the performance was different (Table 3).
Spatiotemporal changes in aerobic and anaerobic bacteria
Aerobic bacteria were most active during April, and PLFA levels were significantly higher than those of other periods in the same soil layer. However, the PLFA content did not change significantly from August to December. Temporal variations were observed in PLFA contents of anaerobic bacteria at depths < 20 cm, with a significant decrease in August, a significant increase in October, and no significant decreases in December; other temporal changes were the same as those of aerobic bacteria (Fig. 5).
In April, the PLFA levels of both types of bacteria showed obvious surface aggregation, and bacteria showed obvious changes in vertical profiles with increasing depth. Aerobic bacteria in layers < 20, 20–40, and 40–100 cm still showed strong interlaminar changes from August to December. However, the PLFA contents of anaerobic bacteria only showed apparent aggregation at depths < 20 cm. Differences between lower layers were not obvious (Fig. 5).
The PLFA ratios for aerobic to anaerobic bacteria were 8.4–21.0, and there were no obvious trends in profile changes. The changes in < 40 cm layers during different periods were substantial; however, the ratio in the 60–100 cm layer was stable, and there were no significant changes (Table 3).
Analysis of factors influencing soil microbial communities
Detrended correspondence analysis of microbial community PLFAs in different months show that the maximum gradient length was 0.96, and linear characteristics were obvious. Therefore, linear model RDA was used to analyse the correlations between microbial community structures and environmental factors. RDA showed that the first and second axes in Fig. 6 explained 43.7% and 21.8% of the community structure variation, respectively, and the first four axes collectively explained 80.5%. The species-environment correlation coefficients of the first two sorting axes were 0.87 and 0.92, respectively, indicating that changes in these nine soil environmental factors were the main factors affecting microbial community structure. In addition to soil temperature, particle contents < 0.001 mm and 0.05–1 mm, the remaining soil physicochemical properties, and other environmental factors significantly affected the microbial communities. Overall, litter and rainfall amounts and field water capacity had the greatest influence on microbial communities, accounting for more than 35% of the total variability (P < 0.05).
Redundancy analysis (RDA) of relationships among microbial community structures, physicochemical properties, and environmental factors. Notes: B, F, AC, G+ , G−, AE, AN, F/B, G+/G−, and AE/AN represent bacteria, fungi, actinomycetes, gram-positive bacteria, gram-negative bacteria, aerobic bacteria, anaerobic bacteria, bacteria/fungi, gram-positive bacteria/gram-negative bacteria, aerobic bacteria/anaerobic bacteria, respectively. SOC, pH, FC, T, PRCP, and TL represent soil organic carbon, pH, field capacity, soil temperature, rainfall, and litter volume, respectively. SMC: soil mechanical composition; SMC1, SMC2, and SMC3 indicate soil particle contents of less than 0.001 mm, 0.01–0.05 mm, and 0.05–1 mm, respectively
Discussion
In this study, litter content, rainfall, soil field capacity, pH, and organic carbon content were important factors affecting microbial community structure. Litter was the primary carbon source and the most important factor. Studies have shown that these factors affect physicochemical properties and alter the microenvironment in which microorganisms live to modulate the activity, distribution, and community structures of microorganisms. Through these mechanisms, microbial PLFA content is altered (Hansson et al. 2011; Hoogmoed et al. 2014; Chen et al. 2015a, b; Banerjee et al. 2016; Deng et al. 2016; Kang et al. 2018; Xu et al. 2018). Changes in 0.01–0.05 mm particle levels due to aeration and water retention also affected microbial community structure.
Soil pH was between 5 and 6 and such acidic conditions are not conducive to bacterial activity. However, some studies have suggested that in a subtropical monsoon climate, litter can rapidly decompose in a high-heat, high-humidity environment, promoting the growth and reproduction of bacteria (Cao et al. 2010), resulting in high levels of PLFAs. Additionally, acidic conditions are more conducive to the activity of fungi than actinomycetes (Chen et al. 2016), and the physiological functions of fungi are related to the decomposition of barely decomposable cellulose and lignin (Zhou et al. 2017a). Therefore, the PLFA contents of soil fungi were lower than those of bacteria but higher than those of actinomycetes. March and April are the peak litter fall times for C. fargesii forests, accounting for 31% of the total annual litter and increasing rainfall, thereby stimulating the growth and reproduction of bacteria, fungi, and actinomycetes. The PLFA contents were significantly higher than in August, October, and December. The decomposition of litter in subtropical evergreen broad-leaved forests was the fastest during the first 90 days, with a weight loss rate of 13.6–20.8%; the rate of weight loss decreased thereafter (Zhao et al. 1991). G+/G− and aerobic/anaerobic bacteria are two bacterial classifications. Our results show that the PLFA contents of soil G+ bacteria or soil aerobic bacteria were much higher than those of G− bacteria and anaerobic bacteria, accounting for total PLFA amounts of 83% and 68%. Therefore, during the early stages of rapid decomposition of litter, soil G+ and aerobic bacteria were the main groups involved in decomposition. With the breakdown of easily decomposable organic substances, relative accumulation of difficult-to-decompose substances such as lignin and cellulose was observed. In August, the decomposition rate of litter was reduced. In addition, nutrient competition between microbes, the growth of trees, and water stress caused by decreased rainfall also affected these results. The PLFA contents of all types of microorganisms decreased significantly. Litter contents also peaked in October; however, due to the high proportion of hard-to-decompose nut fruits (Chang-Yang et al. 2013) and forest growth, the demand for nutrients was reduced, and rainfall was low. The PLFA content of each microorganism was not dramatically affected, although the effects on fungi were significant. December was a period of dormancy. Additionally, the lower soil temperatures and soil water contents caused slow transformation of nutrients, and the growth of microorganisms was limited.
Soil F/B value in April, was 0.1–0.3, and the bacterial community was obviously dominant. With the continuous breakdown of easily decomposed organic matter, the relative accumulation of decay-resistant materials such as lignin and cellulose occurred in August and October. The value of F/B increased to 0.3–0.5, enhancing the fungi influence. The second litter apoptosis peak occurred in December, leading to a significant increase in bacterial dominance in the < 40 cm layer, with little impact on lower layers. Soil F/B values can also be used to evaluate the stability of soil ecosystems, and higher ratios are associated with greater stability (Boyle et al. 2008). The composition of soil microbial communities is greatly influenced by environmental factors and small changes in the soil will cause significant changes in the community (Lopez-Sangil et al. 2011), which will affect the stability of the soil system. Based on litter decomposition, April was the peak period, with complex organic composition and different decomposition characteristics, making the soil microorganisms extremely active. However, the soil ecosystem was in a highly unstable state. As the decomposition rates increased, the organic components were relatively singular, and it was more difficult for lignin and cellulose to decompose, making the soil ecological system more stable.
Compared with soil G− bacteria, soil G+ bacteria had stronger population resistance to pressure and unique mechanisms for adapting to an acidic environment (Zhang et al. 2013b; Yan et al. 2014). Accordingly, G+ bacteria became the main soil bacterial species. Additionally, aerobic bacteria depend on soil aeration. In this study, the soil was sandy, with sand contents 0.05–1 mm reaching as high as 66–73%; deeper soil layers showed higher sand content. The whole profile exhibited good aeration and water permeability, which was beneficial to the multiplication activity of aerobic bacteria, making their PLFA content higher than that of anaerobic bacteria. Studies have shown that G+/G− bacteria ratios can reflect the consumption and accumulation of soil nutrients; higher ratios were related to faster consumption of nutrients, which was obviously not conducive to accumulation (Moore-Kucera and Dick 2008). The surface soil G+/G− bacteria ratios in April and August were significantly higher than in October and December, suggesting that nutrient levels were low. Although the rapid decomposition of litter in April could provide abundant nutrients, this was also the most active time for microbial activity in the year and the growing period for trees. However, large demands for nutrients were not conducive to accumulation in the soil. August was a period of rapid tree growth and the demand for nutrients was high, which was also not conducive to nutrient accumulation. Notably, the G+/G− value in this study was much higher than those reported in other studies (Chang and Chiu 2015; Xiao et al. 2018). The results in a study by Fanin et al. (2013) suggest that P-driven stoichiometric control is important for understanding the spatiotemporal heterogeneity of the G+/G− value and that available soil phosphorus is an important limiting factor affecting soil G− biomass. In this study, available P at the study site was very low (0.5–2.2 mg kg−1) (Wang et al. 2006), which may be an important factor leading to higher G+/G− value. However, further studies are needed to determine the specific reasons for these observations.
In addition to G− bacteria, the PLFA content of soil microorganisms showed obvious surface aggregation. Large amounts of litter, roots, and their secretions provided the soil surface of the C. fargesii forest a sufficient carbon source, rich nutrients, and good ventilation, conducive to the reproduction and activity of soil microorganisms. However, the nature of the subsoil was similar to the parent material, with less organic matter content, poor nutrient status, and moisture and temperature conditions that were not suitable for microbial survival and growth (Pauli et al. 2012; Pasquini et al. 2015).
Conclusion
Soil bacteria, fungi, and actinomycetes were mainly concentrated in the < 20 cm layer, and surface aggregation was obvious. The initial plant growth in April was the most active time for the three types of soil microorganisms; however, the ecosystem was highly unstable. In August during the vigorous growth period, due to the small amount of litter, the inability of organic matter to decompose, the limited rainfall, and the high demand for nutrients in tree growth caused the PLFA contents of the microorganisms to decrease significantly until October (fruit maturity period), and in December (dormant period) to remain stable. Among G+ versus G− bacteria and aerobic versus anaerobic bacteria, G+ and aerobic bacteria were the main bacterial populations influencing overall trends in soil bacterial PLFA levels s and determining the characteristics of spatiotemporal changes in soil bacteria. The dominant microbial population during each time period was bacteria, indicating that soil nutrients were abundant. However, due to the relative accumulation of lignin, cellulose, and other difficult-to-decompose substances, the fungi effect may increase significantly. The soils of the C. fargesii forest were not conducive to the accumulation of nutrients, particularly during the early stages of growth and vigorous growth, during which nutrient contents were relatively low. Overall, the structure of the soil microbial community was affected by many factors, particularly the amounts of litter and rainfall, and soil field capacity. These results provide important information into the changes in soil microbial communities during different seasons in C. fargesii forests and could provide insights into the roles of soil microorganisms in maintaining the health of forests.
References
Banerjee S, Helgason B, Wang L, Winsley T, Ferrari BC, Siciliano SD (2016) Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol Biochem 95:40–50
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515(7528):505–511
Bardgett RD, Hobbs PJ, Frostegard A (1996) Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fert Soils 22(3):261–264
Bossio DA, Fleck JA, Scow KM, Fujii R (2006) Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol Biochem 38(6):1223–1233
Boyle SA, Yarwood RR, Bottomley PJ, Myrold DD (2008) Bacterial and fungal contributions to soil nitrogen cycling under Douglas- fir and red alder at two sites in Oregon. Soil Biol Biochem 40(2):443–451
Breulmann M, Schulz E, Weisshuhn K, Buscot F (2012) Impact of the plant community composition on labile soil organic carbon, soil microbial activity and community structure in semi-natural grassland ecosystems of different productivity. Plant Soil 352(1–2):253–265
Cao Y, Fu S, Zou X, Cao H, Shao Y, Zhou L (2010) Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. Eur J Soil Biol 46(2):128–135
Chang E, Chiu C (2015) Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl Soil Ecol 91:1–7
Chang-Yang C, Lu C, Sun IF et al (2013) Flowering and fruiting patterns in a subtropical rain forest, Taiwan. Biotropica 45(2):165–174
Chen D, Mi J, Chu P, Cheng J, Zhang L, Pan Q, Xie Y, Bai Y (2015a) Patterns and drivers of soil microbial communities along a precipitation gradient on the Mongolian Plateau. Landsc Ecol 30(9):1669–1682
Chen X, Wang D, Chen X, Wang J, Diao J, Zhang J, Guan Q (2015b) Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl Soil Ecol 92:35–44
Chen Y, Ding J, Peng Y, Li F, Yang G, Liu L, Qin S, Fang K, Yang Y (2016) Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J Biogeogr 43(10):2027–2039
Contosta AR, Frey SD, Cooper AB (2015) Soil microbial communities vary as much over time as with chronic warming and nitrogen additions. Soil Biol Biochem 88:19–24
Deng Q, Cheng X, Hui D, Zhang Q, Li M, Zhang Q (2016) Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci Total Environ 541:230–237
Fang J, Barcelona MJ, Alvarez PJJ (2000) A direct comparison between fatty acid analysis and intact phospholipid profiling for microbial identification. Org Geochem 31(9):881–887
Fanin N, Fromin N, Buatois B, Haettenschwiler S (2013) An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant littermicrobe system. Ecol Lett 16(6):764–772
Gong SX, Wang D, Dai W, An XJ, Liu HY (2015) Content and mineralization characteristics of soil organic carbon under Castanopsis fargesii forests in different growth periods. Bull Soil Water Conserv 35(05):59–63
Hansson K, Olsson BA, Olsson M, Johansson U, Kleja DB (2011) Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. For Ecol Manag 262(3):522–530
Hoogmoed M, Cunningham SC, Baker P, Beringer J, Cavagnaro TR (2014) N-fixing trees in restoration plantings: effects on nitrogen supply and soil microbial communities. Soil Biol Biochem 77:203–212
Huang Y, Liu D, An S (2015) Effects of slope aspect on soil nitrogen and microbial properties in the Chinese Loess region. Catena 125:135–145
Huang Y, Wang Y, Liu J, Wang L, Tanaka T, Chen Y, Lu F, Li D (2017) Phenolic compounds from the leaves of Castanopsis fargesii. Molecules 22(1):162
Huygens D, Schouppe J, Roobroeck D, Alvarez M, Balocchi O, Valenzuela E, Pinochet D, Boeckx P (2011) Drying-rewetting effects on N cycling in grassland soils of varying microbial community composition and management intensity in south central Chile. Appl Soil Ecol 48(3):270–279
Kang H, Gao H, Yu W, Yi Y, Wang Y, Ning M (2018) Changes in soil microbial community structure and function after afforestation depend on species and age: case study in a subtropical alluvial island. Sci Total Environ 625:1423–1432
Kourtev P, Ehrenfeld J, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83(11):3152–3166
Li C, Sun Y, Huang HW, Cannon CH (2014) Footprints of divergent selection in natural populations of Castanopsis fargesii (Fagaceae). Heredity 113(6):533–541
Li NJ, Zheng QP, He BH, Zhou F (2017) Seasonal variations of soil microbial PLFAs and Soil enzyme activity under the citrus plantation in Mt. Jinyun, Chongqing. Chin J Environ Sci 01:309–317
Lopez-Sangil L, Rousk J, Wallander H, Casals P (2011) Microbial growth rate measurements reveal that land-use abandonment promotes a fungal dominance of SOM decomposition in grazed Mediterranean ecosystems. Biol Fert Soils 47(2):129–138
McGuire KL, Zak DR, Edwards IP, Blackwood CB, Upchurch R (2010) Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest. Oecologia 164(3):785–795
Moche M, Gutknecht J, Schulz E, Langer U, Rinklebe J (2015) Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol Biochem 90:169–178
Moon JB, Wardrop DH, Bruns MAV, Miller RM, Naithani KJ (2016) Land-use and land-cover effects on soil microbial community abundance and composition in headwater riparian wetlands. Soil Biol Biochem 97:215–233
Moore-Kucera J, Dick RP (2008) PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microb Ecol 55(3):500–511
Nielsen UN, Ball BA (2015) Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob Change Biol 21(4):1407–1421
Pasquini SC, Wright SJ, Santiago LS (2015) Lianas always outperform tree seedlings regardless of soil nutrients: results from a long-term fertilization experiment. Ecology 96(7):1866–1876
Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Benito Alonso JL, Coldea G, Dick J, Erschbamer B, Fernandez Calzado R, Ghosn D, Holten JI, Kanka R, Kazakis G, Kollar J, Larsson P, Moiseev P, Moiseev D, Molau U, Molero Mesa J, Nagy L, Pelino G, Puscas M, Rossi G, Stanisci A, Syverhuset AO, Theurillat J, Tomaselli M, Unterluggauer P, Villar L, Vittoz P, Grabherr G (2012) Recent plant diversity changes on Europe’s Mountain Summits. Science 336(6079):353–355
Richter A, Schoening I, Kahl T, Bauhus J, Ruess L (2018) Regional environmental conditions shape microbial community structure stronger than local forest management intensity. Forest Ecol Manag 409:250–259
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H (2013) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem 57:204–211
State Forestry Bureau (1999) Forest soil analysis methods. China Standard Press, Beijing
Stevenson BA, Hunter DWF, Rhodes PL (2014) Temporal and seasonal change in microbial community structure of an undisturbed, disturbed, and carbon-amended pasture soil. Soil Biol Biochem 75:175–185
Thoms C, Gattinger A, Jacob M, Thomas FM, Gleixner G (2010) Direct and indirect effects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol Biochem 42(9):1558–1565
Wang XQ, Dai W, Xia LF, Deng ZF, Yu HX, Nie LS (2006) Effects of different subtropical plantations on physical and chemical properties of soil. J Beijing For Univ 06:56–59
Wang Q, Gao C, Guo L (2011) Ectomycorrhizae associated with Castanopsis fargesii (Fagaceae) in a subtropical forest, China. Mycol Prog 10(3):323–332
Wang C, Wang G, Wang Y, Rafique R, Ma L, Hu L, Luo Y (2015) Urea addition and litter manipulation alter plant community and soil microbial community composition in a Kobresia humilis meadow. Eur J Soil Biol 70:7–14
Xiao S, Zhang Z, You W, Liu J, Wu J, Cai C, Wu L, Ji Z, He D (2018) Soil microbial community composition in Four&IT Nothotsuga longibracteata&IT forests in Southern China. Pol J Environ Stud 27(2):917–925
Xu Z, Yu G, Zhang X, He N, Wang Q, Wang S, Xu X, Wang R, Zhao N (2018) Divergence of dominant factors in soil microbial communities and functions in forest ecosystems along a climatic gradient. Biogeosciences 15(4):1217–1228
Yan H, Huang YM, Jang YL, Zhao T (2014) Seasonal variation of PLFA during soil mineralization under two kinds of shrub lands in mountainous area of southern Ningxia, Northwest China. Acta Sci Circumst 08:2111–2120
Yao X, Zhang N, Zeng H, Wang W (2018) Effects of soil depth and plant-soil interaction on microbial community in temperate grasslands of northern China. Sci Total Environ 630:96–102
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29(2):111–129
Zhang B, Liang C, He H, Zhang X (2013a) Variations in soil microbial communities and residues along an altitude gradient on the Northern Slope of Changbai Mountain, China. PLoS ONE 8(6):e661846
Zhang C, Liu G, Xue S, Xiao L (2013b) Effect of different vegetation types on the rhizosphere soil microbial community structure in the Loess Plateau of China. J Integr Agric 12(11):2103–2113
Zhang W, Lu Z, Yang K, Zhu J (2017) Impacts of conversion from secondary forests to larch plantations on the structure and function of microbial communities. Appl Soil Ecol 111:73–83
Zhao QG, Wang MZ, He YQ (1991) Litter and its effects on soil in tropical subtropical forests in China. Soils 01:8–15
Zhou Z, Wang C, Jiang L, Luo Y (2017a) Trends in soil microbial communities during secondary succession. Soil Biol Biochem 115:92–99
Zhou Z, Wang C, Zheng M, Jiang L, Luo Y (2017b) Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol Biochem 115:433–441
Acknowledgements
We would like to thank the Chinese Academy of Forestry for supporting the work with funds provided by the Dagangshan Mountain Forest Eco-system Research Station and for assistance with the field work. We would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by the Science and Technology Basic Work Special (No 2014FY120700).
The online version is available at http://www.springerlink.com
Corresponding editor: Chai Ruihai.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Qiao, H., Luan, Y., Wang, B. et al. Analysis of spatiotemporal variations in the characteristics of soil microbial communities in Castanopsis fargesii forests. J. For. Res. 31, 1975–1984 (2020). https://doi.org/10.1007/s11676-019-00957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00957-2