Abstract
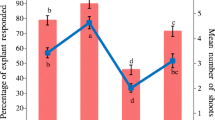
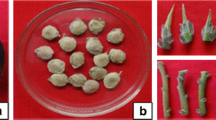
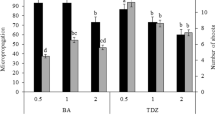
A protocol for micropropagation using nodal explants from mature Pinus massoniana trees has been developed. Time of explant collection is crucial for the initial success of aseptic culture. Explants collected in early March gave the highest percentage of explant survival (64.5%) and shoot-forming percentage (52.3%). Thidiazuron (TDZ) concentration significantly influenced shoot formation; 4 μM TDZ was optimum, with 4.8 shoots produced per explant with a mean length of 7.1 cm after 120 days of culture. Regenerated shoots rooted for 60 days in basic medium with 1 μM NAA were ready for growth in pots. This is the first report on plantlet regeneration in vitro from mature trees of P. massoniana that provides a reliable method for propagating selected elites.


Similar content being viewed by others
References
Ahmad N, Anis M (2007) Rapid plant regeneration protocol for cluster bean (Cyamopsis tetragonoloba L. Taub.). J Hortic Sci Biotechnol 82:585–589
Ahmad N, Siddique I, Anis M (2006) Improved plant regeneration in Capsicum annuum from nodal segment. Biol Plant 50:701–704
Alonso P, Moncaleán P, Fernández B, Rodríguez A, Centeno ML, Ordás R (2006) An improved micropropagation protocol for stone pine (Pinus pinea L.). Ann For Sci 63:879–885
Andersone U, Ievinsh G (2002) Changes of morphogenic competence in mature Pinus sylvestris L. buds in vitro. Ann Bot 90:293–298
Campbell MM, Brunner AM, Jones HM, Strauss SH (2003) Forestry’s fertile crescent: the application of biotechnology to forest trees. Plant Biotechnol J 1:141–154
Capelle SC, Mok DWS, Kirchner SC, Mok MC (1983) Effects of thidiazuron on cytokinin autonomy and the metabolism of N6 (2-isopentenyl)[8-′4C] adenosine in callus tissue of Phaseolus lunatus L. Plant Physiol 73:796–802
Chang YC, Lee N (1992) Tissue culture of Pleione formosana Hayata. J Chin Soc Hortsci 38:80–90
Chen TY, Chen JT, Chang WC (2004) Plant regeneration through direct shoot bud formation from leaf cultures of Paphiopedilum orchids. Plant Cell Tissue Organ Cult 76:11–15
Davis JM, Becwar MR (2007) Examples of current implementation of cloning technology worldwide. In: Page A (ed) Developments in tree cloning. Pira International Ltd, Leatherhead, pp 32–33
De Diego N, Montalbán IA, Fernández E, Moncaleán P (2008) In vitro regeneration of Pinus pinaster adult trees. Can J For Res 38:2607–2615
De Diego N, Montalbán IA, Moncaleán P (2010) In vitro regeneration of adult Pinus sylvestris L. trees. S Afr J Bot 76:158–162
Ding GJ, Zhou ZC, Wang ZR et al (2006) Cultivation and Utilization of Pulpwood Stand for Pinus massoniana[M]. China Forestry Press, Beijing, p 307
Dumas E, Monteuuis O (1995) In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tissue Organ Cult 40:231–235
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Ebert A, Taylor F, Blake J (1993) Changes of 6-benzylaminopurine and 2, 4 dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 33:157–162
Evers PW, Donkers J, Prat A, Vermeer E (1988) Micropagation of forest trees through tissue culture. Pudoc Wageningen, Netherlands, p 84
Faisal M, Ahmad N, Anis M (2005) Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Hohtola A (1988) Seasonal changes in explant viability and contamination of tissue culture from mature Scots pine. Plant Cell Tissue Organ Cult 15:211–222
Hu DN, Shang-guan XC, Liu LY (2009) In vitro regeneration from stem segments of Cyclocarya paliurus (Batal.) Iljinskaja. Hubei Agric Sci 6:1300–1303
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kanyand N, Dessai AP, Prakash SC (1994) Thidiazuron promotes high frequency regeneration of peanut (Arachis hypogea) plants in vitro. Plant Cell Rep 14:1–5
Kartsonas E, Papafotiou M (2007) Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tissue Organ Cult 90:111–116
Khan MI, Ahmad N, Anis M (2011) The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb.: a tree of ecological importance. Trees 25:577–584
Kumar R, Sharma K, Agrawal V (2005) In vitro clonal propagation of Holarrhena antidysenterica (L.) Wall. through nodal explants from mature trees. In Vitro Cell Dev Biol Plant 41:137–144
Laukkanen H, Rautiainen L, Taulavuori E, Hohtola A (2000) Changes of cellular structures and enzymatic activities during browning of Scots pine callus derived from mature buds. Tree Physiol 20:467–475
Lee N, Teng WL (1987) Endogenous growth promoter and inhibitor levels in Pleione formosana corms. Acta Hortic 205:225–231
Lelu-Walter MA, Bernier-Cardou MB, Klimaszewska K (2008) Clonal plant production from self- and cross-pollinated seed families of Pinus sylvestris (L.) through somatic embryogenesis. Plant Cell Tissue Organ Cult 92:31–45
Lu CY (1993) The use of thidiazuron in tissue culture. In vitro Cell Dev Biol Plant 29:92–96
Merkle SA, Dean JF (2000) Forest tree biotechnology. Curr Opin Biotechnol 11:298–302
Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T (1982) Cytokinin activity of N-phenyl-N′-1,2,3-thidiazol-5-ylurea (thidiazuron). Phytochemistry 21:1509–1511
Moncaleán P, Alonso P, Centeno ML, Cortizo M, Rodríguez A, Fernández B, Ordás R (2005) Organogenic response of Pinus pinea cotyledons to hormonal treatments: BA metabolism and cytokinin content. Tree Physiol 25:1–9
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Saxena PK (2001) Molecular fate of thidiazuron and its effects on auxin transport in hypocotyls tissues of Pelargonium x hortorum Bailey. Plant Growth Regul 35:269–275
Pan MJ, Van Staden J (1998) The use of charcoal in in vitro culture—a review. Plant Growth Regul 26:155–163
Pirttilä AM, Laukkanen H, Hohtola A (2002) Chitinase production in pine callus (Pinus sylvestris L.): a defense reaction against endophytes? Planta 214:848–852
Saklani K, Singh S, Purohit VK, Prasad P, Nautiyal AR (2015) In vitro propagation of Rudraksha (Elaeocarpus sphaericus (Gaertn.) K. Schum): a biotechnological approach for conservation. Physiol Mol Biol Plants 21(4):611–615
Shekhawat MS, Manokari M (2016) In vitro propagation, micromorphological studies and ex vitro rooting of cannon ball tree (Couroupita guianensis aubl.): a multipurpose threatened species. Physiol Mol Biol Plants 22(1):131–142
Siddique I, Anis M (2007) In vitro shoot multiplication and plantlet regeneration from nodal explants of Cassia angustifolia: a medicinal plant. Acta Physiol Plant 29:333–338
Stojicic D, Budimir S (2002) Cytokinin-mediated axillary shoot formation in Pinus heldreichii. Biol Plant 48:477–479
Victor JMR, Murthy BNS, Murch SJ, Krihnaraj S, Saxena PK (1999) Role of endogenous purine metabolism in thidiazuron-induced somatic embryogenesis of peanut (Arachis hypogea). Plant Growth Regul 28:41–47
Von Aderkas P, Bonga JM (2000) Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol 20:921–928
Yao RL, Wang Y, Wu YM (2016) Key factors affecting rooting of Pinus massoniana by tissue culture. Guihaia 36(11):1288–1294
Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR (2010) Micropropagation of Pinus massoniana and mycorrhiza formation in vitro. Plant Cell Tissue Organ Cult 102(1):121–128
Acknowledgements
This study was funded by the Science Research and Technology Development from the Department of Science and Technology of Guangxi (14125008-2-17, 1598006-5-7), the Natural Science Foundation of China (31360178), the Key Program of Guangxi Forestry Bureau ([2015]7), and as an independent project by the Key Laboratory of Gaungxi Fine Timber Forest Resources Cultivation (13A-01-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Project funding: This work funded by the Science Research and Technology Development from the Department of Science and Technology of Guangxi (14125008-2-17, 1598006-5-7), the Natural Science Foundation of China (31360178), the Key Program of Guangxi Forestry Bureau ([2015]7), and as an independent project by the Key Laboratory of Gaungxi Fine Timber Forest Resources Cultivation (13A-01-01).
The online version is available at http://www.springerlink.com
Corresponding editor: Chai Ruihai.
Rights and permissions
About this article
Cite this article
Wang, Y., Yao, R. Plantlet regeneration of adult Pinus massoniana Lamb. trees using explants collected in March and thidiazuron in culture medium. J. For. Res. 28, 1169–1175 (2017). https://doi.org/10.1007/s11676-017-0412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0412-9