Abstract
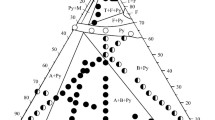
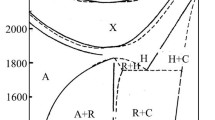
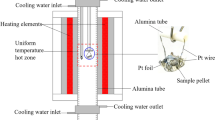
This article considers the features and fundamental difficulties of studying low-temperature phase equilibria associated with an exponential increase in the required duration of syntheses with a decrease in temperature. Methods for accelerating the achievement of equilibrium, including the use of salt solvents, are also considered. The results of phase equilibria studies in the SrF2–LaF3 system using sodium nitrate and in the ZrO2–Sc2O3 system using sodium sulfate as fluxes are presented. The methods of extrapolation of phase diagrams to absolute zero temperature in accordance with the third law of thermodynamics are considered. Phase diagrams of the Au–Cu, Cu–Pd, Ni–Pt, and ZrO2–Y2O3 systems are presented. Phase equilibria with plagioclase ordering are considered separately, and the phase diagram of the albite–anorthite (NaAlSi3O8–CaAl2Si2O8) system is presented. As the temperature approaches absolute zero, the homogeneity region of labradorite shrinks to the compound NaCaAl3Si5O16.






Copyright© 2024 The American Ceramic Society, all rights reserved.








Similar content being viewed by others
References
T.B. Massalski, Phase Diagrams in Materials Science, Metall. Trans., 1989, 20A, p 1295-1323.
J.P. Abriata and D.E. Laughlin, The Third Law of Thermodynamics and Low Temperature Phase Stability, Prog. Mater. Sci., 2004, 48(3), p 367-387.
D.E. Laughlin and W.A. Soffa, The Third Law of Thermodynamics: Phase Equilibria and Phase Diagrams at Low Temperatures, Acta Mater., 2018, 45, p 49-61.
P.P. Fedorov, Anneal Time Determined by Studying Phase Transitions in Solid Binary Systems, Russ. J. Inorg. Chem., 1992, 37(8), p 968-973.
P.P. Fedorov, Third Law of Thermodynamics as Applied to Phase Diagrams, Russ. J. Inorg. Chem., 2010, 55(11), p 1722-1739. https://doi.org/10.1134/S0036023610110100
P.P. Fedorov and E.V. Chernova, The Conditions for the Solid State Synthesis of Solid Solutions in Zirconia and Hafnia Systems with the Oxides of Rare Earth Elements, Condens. Matter Interphases., 2022, 24(4), p 537-544. https://doi.org/10.17308/kcmf.2022.24/10558
F.M. Spiridonov, L.N. Popova, and R. Ya, Popil’skii, On the Phase Relations and Electrical Conductivity in the System ZrO2-Sc2O3, J. Solid State Chem., 1970, 2, p 430-438.
C. Pascual and P. Duran, Subsolidus Phase Equilibria and Ordering in the System ZrO2-Y2O3, J. Am. Ceram. Soc., 1983, 66, p 23-28.
V.S. Stubican, G.S. Corman, J.R. Hellmann, G. Sent, Phase Relationships in Some ZrO2 System Advanced in Ceramics. Science and Technology of Zirconia. II. Ed. N. Clausen, M. Rühle, A. Heuer, Columbus, OH, 1984, 12, p 96-106.
M. Yashima, M. Kakihana, and M. Yoshimura, Metastable-Stable Phase Diagrams in the Zirconia-Containing Systems Utilized in Solid-Oxide Fuel Cell Application, Solid State Ionics, 1996, 86, p 1131-1149.
A.A. Popov, A.D. Varygin, P.E. Plyusnin, M.R. Sharafutdinov, S.V. Korenev, A.N. Serkova, and Yu.V. Shubin, J. Alloys Compd., 2021, 891, p 161974. https://doi.org/10.1016/j.jallcom.2021.161974
P.P. Fedorov, A.A. Alexandrov, V.V. Voronov, M.N. Mayakova, A.E. Baranchikov, and V.K. Ivanov, Low-Temperature Phase Formation in the SrF2 - LaF3 System, J. Am. Ceram. Soc., 2021, 104(6), p 2836-2848. https://doi.org/10.1111/jace.17666
P.P. Fedorov, Phase Diagrams of Lead Difluoride Systems with Rare-Earth Fluorides, Russ. J. Inorg. Chem., 2021, 66(2), p 245-252. https://doi.org/10.1134/S0036023621020078
Y. Igami, S. Ohi, and A. Miyake, Sillimanite-Mullite Transformation Observed in Synchrotron X-ray Diffraction Experiments, J. Am. Ceram. Soc., 2017, 100, p 4928-4937. https://doi.org/10.1111/jace.15020
V. Manivannan, P. Parhi, and J.W. Kramer, Metathesis Synthesis and Characterization of Complex Metal Fluoride, KMF3 (M = Mg, Zn, Mn, Ni, Cu and Co) Using Mechanochemical Activation, Bull. Mater. Sci., 2008, 31(7), p 987-993.
J. Lee, Q. Zhang, and F. Saito, Synthesis of Nano-Sized Lanthanum Oxyfluoride Powders by Mechanochemical Processing, J. Alloys Comp., 2003, 348, p 214-219.
A.A. Luginina, A.E. Baranchikov, A.I. Popov, and P.P. Fedorov, Preparation of Barium Monohydrofluoride BaF2·HF from Nitrate Aqueous Solutions, Mater. Res. Bull., 2014, 49(1), p 199-205. https://doi.org/10.1016/j.materresbull.2013.08.074
V.V. Gusarov, Fast Solid-Phase Chemical Reactions, Russ. J. Gen. Chem., 1997, 67(12), p 1846-1851.
W. Eysel, Crystal Chemistry of the system Na2SO4-K2SO4-K2CrO4-Na2CrO4 and of Glaserite Phase, Am. Mineral., 1973, 58, p 736-747.
N.V. Shchipalkina, I.V. Pekov, S.N. Britvin, N.N. Koshlyakova, and E.G. Sidorov, Alkali Sulfates with Aphthitalite-like Structures from Fumaroles of the Tolbachik Volcano, Kamchatka, Russia. III. Solid Solutions and Exsolutions, Can. Mineral., 2021, 59, p 713-727. https://doi.org/10.3749/canmin.2000105
M. Liu and R.A. Yund, NaSi-CaAl Interdiffusion in Plagioclase, Am. Mineral., 1992, 77, p 275-283.
M. Yoshimura, K.J. Kim, E. Tani, and S. Somiya, Establishment of the Equilibrium Phase Diagram in Fluorite-Related Systems, ZrO2-CeO2 and SrF2–LaF3, by Hydrothermal Technique, Solid State Ionics, 1988, 28-30, p 452-457.
X. Xia, A. Pant, X. Zhou, E.A. Dobretsova, A.B. Bard, M.B. Lim, J.Y.D. Roh, D.R. Gamelin, and P.J. Pauzauskie, Hydrothermal Synthesis and Solid-State Laser Refrigeration of Ytterbium-Doped Potassium-Lutetim-Fluoride (KLF) Microcrystals, Chem. Mater., 2021, 33, p 4417-4424.
J. Warshay and R. Roy, Polymorphism of the Rare Earth Sesquioxides, J. Phys. Chem., 1961, 65, p 2048.
J.-P. Traverse, Contribution au d”eveloppement de méthodes d’expérimentation à température élevée. Application à l’étude du polymorphisme des sequioxydes de terres rares et des changements de phases dans les systèmes zircone-chaux et zircone-oxyde de strontium (Contribution to the development of high temperature experimental methods. Application to the study of polymorphism of rare earth sequioxides and phase changes in zirconia-lime and zirconia-strontium oxide systems), Thesis. Grenoble: L’Université Scientifique et Medicale de Grenoble, 1971, 150 p.
C. Boulesteix, Handbook on the Physics and Chemistry of Rare Earth, Eds Gschneidner K.A., Eyring L. Amsterdam: North-Holland Publ. Company, 1982, Ch. 44, p. 321.
V.B. Glushkova, Polymorphism of Rare-Earth Oxides. Nauka, Leningrad, 1967. , (in Russian)
P.P. Fedorov, M.V. Nazarkin, and R.M. Zakalyukin, On Polymorphism and Morphotropism of Rare-Earth Sesquioxides, Crystallogr. Rep., 2002, 47(2), p 281-286. https://doi.org/10.1134/1.1466504
P.P. Fedorov and A.A. Alexandrov, Synthesis of Inorganic Fluorides in Molten Salt Fluxes and Ionic Liquid Mediums, J. Fluorine Chem., 2019, 227, p 109374.
P.P. Fedorov, M.N. Mayakova, A.A. Alexandrov, V.V. Voronov, S.V. Kuznetsov, A.E. Baranchikov, and V.K. Ivanov, The Melt of Sodium Nitrate as a Medium for Synthesis of Fluorides, Inorganics, 2018, 6(2), p 38-55. https://doi.org/10.3390/inorganics6020038
P.P. Fedorov, V.Yu. Proydakova, S.V. Kuznetsov, V.V. Voronov, A.A. Pynenkov, and K.N. Nishchev, Phase Diagram of the Li2SO4-Na2SO4 System, J. Am. Ceram. Soc., 2020, 103(5), p 3390-3400. https://doi.org/10.1111/jace.16996
B.P. Sobolev and K.B. Seiranian, Phase Diagrams of the SrF2-(Y, Ln)F3 Systems, J. Solid State Chem., 1981, 39(3), p 337-344. https://doi.org/10.1016/0022-4596(81)90268-1
M. Yoshimura, K.J. Kim, and S. Somiya, Revised Subsolidus Phase Diagram of the System SrF2-LaF3, Solid State Ionics, 1986, 18-19, p 1211-1216. https://doi.org/10.1016/0167-2738(86)90336-X
M.A. Bredig, The Order-Disorder (λ) Transition in UO2 and Other Solids of the Fluorite Type of Structure, Colloq. Int. CNRS, 1972, 205, p 183-197.
J. Eapen and A. Annamareddy, Entropic Crossovers in Superionic Fluorites from Specific Heat, Ionics, 2017, 23, p 1043-1047. https://doi.org/10.1007/s11581-017-2007-z
P.C.M. Fossati, A. Chartier, and A. Boulle, Structural Aspects of the Superionic Transition in AX2 Compounds with the Fluorite Structure, Front. Chem., 2021, 9, p N723507. https://doi.org/10.3389/fchem.2021.723507
T. Sekiya, T. Yamada, H. Hayashi, and T. Noguchi, High Temperature Phase in the ZrO2-Sc2O3 System, J. Chem. Soc. Jpn., 1974, 9, p p1629-1636. (in Japanese)
A.V. Shevchenko, I.M. Maister, and L.M. Lopato, Interaction in the HfO2-Sc2O3 and ZrO2-Sc2O3 Systems at High Temperatures, Neorganicheskie Materialy (Inorganic Materials), 1987, 23(8), p 1320-1324. (in Russian)
A.V. Zyrin, V.P. Redko, L.M. Lopato, A.V. Shevchenko, I.M. Maister, and Z.A. Zaitseva, Ordered Phases in ZrO2-Sc2O3 and HfO2-Sc2O3 Systems, Neorganicheskie materialy (Inorganic Materials), 1987, 23(8), p 1325-1329. (In Russian)
L.M. Lopato, V.P. Redko, G.I. Gerasimuk, and A.V. Shevchenko, Synthesis of Some Compounds of Zirconia and Hafnia with Oxides of REE, Powder Metall. Metal Ceram., 1990, 4, p 73-75. (in Russian)
I.M. Maister, L.M. Lopato, Z.A. Zaitseva, and A.V. Shevchenko, Interaction in the system ZrO2-Y2O3-Sc2O3 at 1300–1900 °C, Neorganicheskie materialy (Inorganic Materials), 1991, 27(11), p 2337-2340.
H. Fujimori, M. Yashima, M. Kakihana, and M. Yoshimura, Structural Changes of Scandia-Doped Zirconia Solid Solutions: Rietveld Analysis and Raman Scattering, J. Am. Ceram. Soc., 1998, 81(11), p 2885-2893.
H. Fujimori, M. Yashima, M. Kakihana, and M. Yoshimura, β-Cubic Phase Transition of Scandia-Doped Zirconia Solid Solution: Calorimetry, X-ray Diffraction, and Raman Scattering, J. Appl. Phys., 2002, 91, p 6493-6498. https://doi.org/10.1063/1.1471576
K. Wurst, E. Schweda, D.J.M. Bevan, J. Mohyla, K.S. Wallwork, and M. Hofmann, Single-Crystal Structure Determination of Zr50Sc12O118, Solid State Sci., 2003, 5, p 1491-1497.
S. Meyer, E. Schweda, N.J.M. Meta, H. Boysen, M. Hoelzel, and T. Bredow, Neutron Powder Diffraction Study and DFT Calculations of the Structure of Zr10Sc4O26, Z. Kristallogr. (J. Crystallogr.), 2009, 224, p 539-543.
M.R. Thornber and D.J.M. Bevan, Mixed Oxides of the Type MO2 Fluorite-M2O3. III Crystal Structures of the Intermediate Phases Zr5Sc2O15 and Zr3Sc2O12, Acta Crystallogr. B, 1968, 24, p 1183-1190.
W. Nernst, The new heat theorem Methuen and Co., London, 1917, p313
F.E. Simon, Some Considerations Concerning Nernst’s Theorem, Z. Naturforschg. (J. Nat. Res.), 1951, 6, p 397-400. https://doi.org/10.1515/zna-1951-0717
B. Hallstedt, Thermodynamic Assessment of the System MgO-Al2O3, J. Amer. Ceram. Soc., 1992, 75(6), p 1497-1507.
D.E. Laughlin and T.B. Massalski, Construction of Equilibrium Phase Diagrams: Some Errors to be Avoided, Prog. Mater. Sci., 2020. https://doi.org/10.1016/j.pmatsci.2020.100715
H. Okamoto, D.J. Chakrabarti, D.E. Laughlin, and T.B. Massalski, The Au− Cu (gold-copper) System, Bull. Alloy Phase Diagrams, 1987, 8(5), p 454-473.
P.P. Fedorov and S.N. Volkov, Au-Cu Phase Diagram, Russ. J. Inorg. Chem., 2016, 61(6), p 772-775. https://doi.org/10.1134/S0036023616060061
N.S. Kurnakov, S.F. Zemchuzny, and M. Zasedatelev, The Transformations in Alloys of Gold with Copper, J. Inst. Met. London, 1916, 15, p 305.
A.A. Popov, Yu.V. Shubin, P.E. Plyusnin, M.R. Sharafutdinov, and S.V. Korenev, Experimental Redetermination of the Cu–Pd Phase Diagram, J. Alloys Compd., 2019, 777, p 204. https://doi.org/10.1016/j.jallcom.2018.10.332
P.P. Fedorov, Yu.V. Shubin, and E.V. Chernova, Cooper-Palladium Phase diagram, Russ. J. Inorg. Chem., 2021, 66(6), p 891-893. https://doi.org/10.1134/S0036023621050053
P.R. Subramanian and D.E. Laughlin, Cu-Pd (Copper-Palladium), J. Phase Equilibria, 1991, 12, p 231-243. https://doi.org/10.1007/BF02645723
B. Schönfeld, M. Engelke, and A.S. Sologubenko, Microstructure and Order in NiPt3, Philos. Mag., 2015, 95(10), p 1080-1092. https://doi.org/10.1080/14786435.2015.1019587
P.P. Fedorov, A.A. Popov, Yu.V. Shubin, and E.V. Chernova, Phase Diagram of the Nickel-Platinum System, Russ. J. Inorg. Chem., 2022, 67(12), p 2018-2022. https://doi.org/10.1134/S0036023622601453
X.-G. Lu, B. Sundman, and J. Ågren, Thermodynamic Assessments of the Ni–Pt and Al–Ni–Pt Systems, Calphad, 2009, 33, p 450-456. https://doi.org/10.1016/j.calphad.2009.06.002
C.E. Dahmani, M.C. Cadeville, J.M. Sanchez, and J.L. Moran-Lopez, Ni-Pt Phase Diagram: Experiment and Theory, Phys. Rev. Lett., 1985, 55(11), p 1208. https://doi.org/10.1103/PhysRevLett.55.1208
L. Landau, Towards the Theory of Phase Transitions, JETP, 1937, 7, p 19-32. (in Russian)
R.B. Griffiths, Proposal for a Notation at Tricritical Points, Phys. Rev. B. J. Chem. Phys., 1973, 7(1), p 545-551.
A. Aharony, Multicritical Points. In: Critical Phenomena, ed. F.J.W. Hahne, Lecture Notes in Physics, Berlin, Heidelberg: Springer, 1983, 186, p 209-258, https://doi.org/10.1007/3-540-12675-9_13
M.A. Anisimov, E.E. Gorodetskii, and V.M. Zaprudskii, Phase Transitions with Coupled Order Parameters, Sov. Phys. Usp., 1981, 24(1), p 57-75. https://doi.org/10.1070/PU1981v024n01ABEH004612
N.E. Phillips, 3He-4He Solutions, Annu. Rev. Phys. Chem., 1968, 19, p 559-584. https://doi.org/10.1146/annurev.pc.19.100168.003015
B.N. Esel’son, V.N. Grigor’ev, V.G. Ivantsov, E. Ya. Rudavskiy, D.G. Sanikadze, I.A. Serbin, Quantum Fluid Solutions 3He-4He, Moscow: Nauka, 1973 (in Russian).
J.R. Goldsmith and H.C. Heard, Subsolidus Phase Relations in the System CaCO3-MgCO3, J. Geol., 1961, 69(1), p 45-74.
P.P. Fedorov, A.A. Alexandrov, S.V. Kuznetsov, V.V. Voronov, Comment on the paper Thermodynamic evaluation and optimization of the (NaNO3 + KNO3 + Na2SO + K2SO4) system by Ch. Robelin, P. Chartrand, A.D. Pelton, published in J. Chem. Therm. 2015, 83, p 12-26, J. Chem. Therm. 2020, 149, p 106178 (7 pp), https://doi.org/10.1016/j.jct.2020.106178
A.A. Smirnov, Molecular kinetic theory of metals, M.: Nauka, 1966 (in Russian).
P.P. Fedorov and E.V. Chernova, Phase Diagrams of Zirconia Systems with Yttria and Scandia, Condens. Matter Interphases, 2023, 25(2), p 257-267. https://doi.org/10.17308/kcmf.2023.25/11106
R.J. Gaboriaud, F. Paumier, and B. Lacroix, Disorder-Order Phase Transformation in a Fluorite-Related Oxide Film: in-situ X-ray Diffraction and Modeling of the Residual Stress Effects, Thin Solid Films, 2016, 601, p 84-88.
P. Duwez, F.H. Brown, and F. Odell, The Zirconia-Yttria System, J. Electrochem. Soc., 1951, 98(9), p 356-362.
M. Yashima, N. Ishizawa, T. Nama, and M. Yoshimura, Stable and Metastable Phase Relationships in the System ZrO2-ErO1.5, J. Am. Ceram. Soc., 1991, 74(3), p 510-513.
M.A. Carpenter, Subsolidus Phase Relations of the Plagioclase Feldspar Solid Solution, Feldspars and their Reactions, ed. I. Parsons, NATO ASI Series C 421, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1994, p 221-269.
J.D.C. McConnell, The Origin and Characteristics of the Incommensurate Structures in the Plagioclase Feldspars, Can. Mineral., 2008, 46, p 1389-1400. https://doi.org/10.3749/canmin.46.6.1389
I. Parson, Feldspars Defined and Described: A Pair of Posters Publishing by the Mineralogical Society. Sources and Supporting Information, Mineral. Mag., 2010, 74, p 529-551. https://doi.org/10.1180/minmag.2010.074.3.529
S.K. Filatov, A.P. Shablinskii, S.N. Volkov, and R.S. Bubnova, Forms of Solid Solution Ordering Upon Decreasing Temperature, J. Struct. Chem., 2017, 58, p 135-158. https://doi.org/10.1134/S0022476617010206
S. Jin and H. Xu, Investigations of the Phase Relations Among e1, e2 and C1 Structures of Na-Rich Plagioclase Feldspars: A Single-Crystal X-Ray Diffraction Study, Acta Cryst., 2017, B73, p 992-1006. https://doi.org/10.1107/S2052520617010976
S. Jin, H. Xu, X. Wang, R. Jacob, and D. Morgan, The Incommensurately Modulated Structures of Low-Temperature Labradorite Feldspars: A Single Crystal X-ray and Neutron Diffraction Study, Acta Cryst., 2020, B76, p 93-107.
D.E. Laughlin and T.B. Massalski, Construction of Equilibrium Phase Diagrams: Some Errors to be Avoided, Prog. Mater. Sci., 2021, 120, p 100715. https://doi.org/10.1016/j.pmatsci.2020.100715
M.A. Carpenter, A “Conditional Spinodal” Within the Peristerite Miscibility Gap of Plagioclase Feldspars, Am. Miner., 1981, 66(5-6), p 553-560.
S. Sole, C. Schmetterer, and K.W. Richter, A Revision of the Sb-Te Binary Phase Diagram and Crystal Structure of the Modulated Phase Field, J. Phase Equilib. Diffus., 2022, 43, p 648-659.
J.H. Muller, T. Petzel, and B. Hormann, Phase Study of the Binary System Lu2O3-LuF3 in the Temperature Range 1000–1750 K, Thermochim. Acta, 1997, 298, p 109-114. https://doi.org/10.1016/S0040-6031(97)00190-1
E.G. Yarotskaya and P.P. Fedorov, Mullite and its Isomorphic Substitution, Condens. Matter Interphases, 2018, 20(4), p 537-544. https://doi.org/10.17308/kcmf.2018.20/626
W.E. Cameron, Mullite; A Substituted Alumina, Am. Mineralog., 1977, 62(7-8), p 747-755.
S. Padlewski, V. Heine, and G.D. Price, A Microscopic Model for a Very Stable Incommensurate Modulated Mineral: Mullite, J. Phys. Condens. Matter, 1993, 5, p 3417-3430. https://doi.org/10.1088/0953-8984/5/21/004
P.P. Fedorov and E.G. Yarotskaya, Zirconium dioxide, Condens. Matter Interphases, 2021, 23(2), p 169-187. https://doi.org/10.17308/kcmf.2021.23/3427
I. Prigogine and R. Defay, Chemical Thermodynamics. Longman Green and Co. London et al., 1954.
M.C. Bhardwaj and R. Roy, Effect of High Pressure on Crystalline Solubility in the System NaCl-KCl, Phys. Chem. Sol., 1971, 32, p 1607-1693.
A. Schiraldi, E. Pezzati, and G. Chiodelli, Phase Diagram and Point Defect Parameters of the System CsBr-TlBr, Z. Phys. Chem. Neue Folge, 1978, 110, p 1-16.
A.A. Eliseev, A.V. Lukashin, and A.A. Vertigel, Cryosol Synthesis of Al2-xCrxO3 Solid Solutions, Chem. Mater., 1999, 11, p 241-246.
P.P. Fedorov, Nanotechnology and Material Science, Nanosyst. Phys. Chem. Math., 2020, 11(3), p 314-315. https://doi.org/10.17586/2220-8054-2020-11-3-314-315
Acknowledgments
The author is deeply grateful to A.A. Alexandrov and V.Yu. Proidakova for conducting the experiments and E.V. Chernova for her help in the preparation of the manuscript. The study was supported by the Russian Science Foundation (project No. 22-13-00167) https://rscf.ru/project/22-13-00167/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited article is part of a special tribute issue of the Journal of Phase Equilibria and Diffusion dedicated to the memory of Thaddeus B. “Ted” Massalski. The issue was organized by David E. Laughlin, Carnegie Mellon University; John H. Perepezko, University of Wisconsin–Madison; Wei Xiong, University of Pittsburgh; and JPED Editor-in-Chief Ursula Kattner, National Institute of Standards and Technology (NIST).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fedorov, P.P. Phase Equilibria in Low-Temperature Regions of Phase Diagrams. J. Phase Equilib. Diffus. (2024). https://doi.org/10.1007/s11669-024-01099-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11669-024-01099-7