Abstract
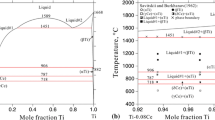
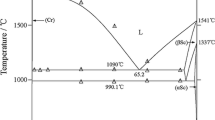
Thermodynamic optimization of the Ln-Ru (where \({\text{Ln}} = {\text{Ce and Sm}}\)) binary systems was carried out by means of CALPHAD method. Our approach is based on the critical review of the available experimental data published in literature, including both phase equilibria and thermodynamic data. Nine intermediate phases:\({\text{Ln}}_{3} {\text{Ru}},\;{\text{Ce}}_{7} {\text{Ru}}_{3} ,\;{\text{Ce}}_{16} {\text{Ru}}_{9} ,\;{\text{Ce}}_{4} {\text{Ru}}_{3} ,\;{\text{Sm}}_{2} {\text{Ru}}_{5} ,\;{\text{Ru}}_{25} {\text{Sm}}_{44} ,\;{\text{and}}\;{\text{LnRu}}_{2}\) presented in both binary systems, were treated as stoichiometric compounds. The exponential and linear models were used to describe the temperature dependence of the excess quantities of liquid, \((\delta {\text{Ce}}),\;(\gamma {\text{Ce}}),\;(\beta {\text{Ce}}),(\alpha {\text{Sm}}),\;(\beta {\text{Sm}}),\;(\gamma {\text{Sm}})\;{\text{and}}\;({\text{Ru}})\) solid solution phases. They were compared with the combined linear–exponential temperature dependence of the resulting interaction energies. The self-consistent thermodynamic parameters used to describe the Gibbs energies of various phases in the Ln-Ru binary systems were obtained through Thermo-Calc software. The obtained calculated results are in excellent agreement with available phase equilibrium and thermodynamic data.








Similar content being viewed by others
References
L. Schlapbach, and A. Züttel, Hydrogen-Storage Materials for Mobile Applications, Nature, 2001. https://doi.org/10.1038/35104634
K.H.J. Buschow, Intermetallic Compounds of Rare-Earth and 3d Transition Metals, Rep. Prog. Phys., 1977, 40, p 1179. https://doi.org/10.1088/0034-4885/40/10/002/meta
K.H.J. Buschow, Permanent Magnet Materials Based on Tetragonal Rare Earth Compounds of the Type RFe12-xMx, J. Magn. Magn. Mater., 1991, 100, p 79–89. https://doi.org/10.1016/0304-8853(91)90813-P
X. Moya, S. Kar-Narayan, and N.D. Mathur, Caloric Materials Near Ferroic Phase Transitions, Nat. Mater., 2014, 13, p 439–450. https://doi.org/10.1038/nmat3951
A.M. Tishin, Chapter 4 Magnetocaloric Effect in the Vicinity of Phase Transitions, Handb. Magn. Mater., 1999, 12, p 395–524. https://doi.org/10.1016/S1567-2719(99)12008-0
N.A. de Oliveira, and P.J. von Ranke, Theoretical Aspects of the Magnetocaloric Effect, Phys. Rep., 2010, 489, p 89–159. https://doi.org/10.1016/j.physrep.2009.12.006
Z. Hou, Q. Zhang, G. Xu, C. Gong, B. Ding, Y. Wang, H. Li, E. Liu, F. Xu, H. Zhang, Y. Yao, G. Wu, X.X. Zhang, and W. Wang, Creation of Single Chain of Nanoscale Skyrmion Bubbles with Record-High Temperature Stability in a Geometrically Confined Nanostripe, Nano Lett., 2018, 18, p 1274–1279. https://doi.org/10.1021/acs.nanolett.7b04900
Z. Hou, Q. Zhang, G. Xu, S. Zhang, C. Gong, B. Ding, H. Li, F. Xu, Y. Yao, E. Liu, G. Wu, X.X. Zhang, and W. Wang, Manipulating the Topology of Nanoscale Skyrmion Bubbles by Spatially Geometric Confinement, ACS Nano, 2019, 13, p 922–929. https://doi.org/10.1021/acsnano.8b09689
M. Balli, S. Jandl, P. Fournier, and A. Kedous-Lebouc, Advanced Materials for Magnetic Cooling: Fundamentals and Practical Aspects, Appl. Phys. Rev., 2017. https://doi.org/10.1063/1.4983612
L. Li, and M. Yan, Recent Progresses in Exploring the Rare Earth Based Intermetallic Compounds for Cryogenic Magnetic Refrigeration, J. Alloys Compd., 2020, 823, p 153810. https://doi.org/10.1016/j.jallcom.2020.153810
J. Gaálová, J. Barbier, and S. Rossignol, Ruthenium Versus Platinum on Cerium Materials in Wet Air Oxidation of Acetic Acid, J. Hazard. Mater., 2010, 181, p 633–639. https://doi.org/10.1016/j.jhazmat.2010.05.059
K. Gunnarsson, and K. Schönhammer, Photoemission from Ce Compounds: Exact Model Calculation in the Limit of Large Degeneracy, Phys. Rev. Lett., 1983, 50, p 604–607. https://doi.org/10.1103/PhysRevLett.50.604
M.D.E. Weschke, C. Laubschat, R. Ecker, A. Hohr, and G. Kaindl, Bandlike Character of 4f Electrons in CeRh3, Phys. Rev. Lett., 1992, 69, p 1792–1795. https://doi.org/10.1103/PhysRevLett.69.1792
T. Takeshita, W.E. Wallace, and R.S. Craig, Rare Earth lntermetallics as Synthetic Ammonia Catalysts, J. Catal., 1976, 44, p 236–243. https://doi.org/10.1016/0021-9517(76)90394-8
R.M. Nix, T. Rayment, R.M. Lambert, J.R. Jennings, and G. Owen, An In Situ X-ray Diffraction Study of the Activation and Performance of Methanol Synthesis Catalysts Derived from Rare Earth-Copper Alloys, J. Catal., 1987, 106, p 216–234. https://doi.org/10.1016/0021-9517(87)90226-0
A.P. Walker, and R.M. Lambert, Properties of the Ru(0001)/Ce-H2 Interface: A Model System for Transition-Metal/Rare-Earth Hydride Catalysts, J. Phys. Chem., 1992, 1, p 2265–2271.
A. Trovarelli, Catalytic Properties of Ceria and CeO2-Containing Materials, Catal. Rev. Sci. Eng., 1996, 38, p 439–520. https://doi.org/10.1080/01614949608006464
N. Saunders, and A.P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide. Elsevier, Amsterdam, 1998.
L. Kaufman, and H. Bernstein, Computer Calculation of Phase Diagrams (With Special Reference to Refractory Metals). Academic Press Inc, New York, 1970.
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics: The Calphad Method. Cambridge University Press, Cambridge, 2007. https://doi.org/10.1017/CBO9780511804137
G. Kaptay, Materials Equilibria in Macro-, Micro-and Nano-systems, Raszter Ny (2011)
R. Schmid-Fetzer, D. Andersson, P.Y. Chevalier, L. Eleno, O. Fabrichnaya, U.R. Kattner, B. Sundman, C. Wang, A. Watson, L. Zabdyr, and M. Zinkevich, Assessment Techniques, Database Design and Software Facilities for Thermodynamics and Diffusion, Calphad, 2007, 31, p 38–52. https://doi.org/10.1016/j.calphad.2006.02.007
G. Kaptay, Nano-Calphad: Extension of the Calphad Method to Systems with Nano-phases and Complexions, J. Mater. Sci., 2012, 47, p 8320–8335. https://doi.org/10.1007/s10853-012-6772-9
N. Selhaoui, J. Charles, L. Bouirden, and J.C. Gachon, Optimization of the Binary Ce–Ru System, J. Alloys Compd., 1998, 269, p 166–172. https://doi.org/10.1016/S0925-8388(98)00125-X
J.C. Gachon, J. Charles, and J. Hertz, Different Ways to Find the Thermodynamic Functions Describing the Formation of Binary Alloys Part 1 Comparison Between Models and Experimental Data, Calphad, 1985, 9, p 29–34. https://doi.org/10.1016/0364-5916(85)90028-8
J. Charles, M. Notin, M. Rahmane, and J. Hertz, NANCYUN: A Laboratory Tool for Calculation of Binary Systems, J. Phase Equilibria, 1992, 13, p 497–506. https://doi.org/10.1007/BF02665762
B. Jansson, Computer Operated Methods for Equilibrium Calculations and Evaluation of Thermochemical Model Parameters. Royal Institute of Technology, Stockholm, 1984.
B. Sundman, B. Jansson, and J.-O. Andersson, The Thermo-Calc Databank System, Calphad Comput., 1985, 9, p 153–190. https://doi.org/10.1016/0364-5916(85)90021-5
S.L. Chen, S. Daniel, F. Zhang, Y.A. Chang, W.A. Oates, and R. Schmid-Fetzer, On the Calculation of Multicomponent Stable Phase Diagrams, J. Phase Equilibria, 2001, 22, p 373–378. https://doi.org/10.1361/105497101770332910
G. Kaptay, A New Equation for the Temperature Dependence of the Excess Gibbs Energy of Solution Phases, Calphad, 2004, 28, p 115–124. https://doi.org/10.1016/j.calphad.2004.08.005
T. Abe, K. Ogawa, and K. Hashimoto, Analysis of Miscibility Gaps Based on the Redlich-Kister Polynomial for Binary Solutions, Calphad, 2012, 38, p 161–167. https://doi.org/10.1016/j.calphad.2012.06.006
G. Kaptay, On the Tendency of Solutions to Tend Toward Ideal Solutions At High Temperatures, Metall. Mater. Trans. A, 2012, 43, p 531–543. https://doi.org/10.1007/s11661-011-0902-x
J.F. Elliott, and C.H.P. Lupis, Correlation Between Excess Entropy and Enthalpy Functions, Trans Metall. Soc. AIME., 1966, 236, p 130.
G. Kaptay, On the Abilities and Limitations of the Linear, Exponential and Combined Models to Describe The Temperature Dependence of the Excess Gibbs Energy of Solutions, Calphad, 2014, 44, p 81–94. https://doi.org/10.1016/j.calphad.2013.08.007
W. Obrowski, Über den Aufbau des Systems Ruthenium-Cer, Int. J. Mater. Res., 1962, 53, p 736–737. https://doi.org/10.1515/ijmr-1962-531108
R.D. Reiswig, and K.A. Gschneidner, Melting Points of LaRu2, CeRu2, and PrRu2, J. Less Common Metals, 1963, 5, p 432–433.
A. Palenzona, The Phase Diagram of the Ce-Ru System, J. Alloys Compd., 1991, 176, p 241–246. https://doi.org/10.1016/0925-8388(91)90031-P
A. Palenzona, and F. Canepa, The Phase Diagrams of the La-Ru and Nd-Ru Systems, J. Less-Common Metals, 1990, 157, p 307–313. https://doi.org/10.1016/0022-5088(90)90186-N
B. Predel, Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys, Landolt-Börnstein—Group IV Physical Chemistry, Vol. 5. Springer, Germany, 1991.
H. Okamoto, Ce-Ru (Cerium-Ruthenium), J. Phase Equilibria, 1992, 13, p 437–438. https://doi.org/10.1007/978-3-540-44756-6_147
T.B. Massalski, H. Okamoto, P. Subramanian, L. Kacprzak, and W.W. Scott, Binary Alloy Phase Diagrams, 2nd edn. ASM International, Materials Park, 1990.
W. Moffatt, The Handbook of Binary Phase Diagrams. General Electric, Schenectady, 1978.
A.L. Shilov, E.I. Yaropolava, M.V. Raevskaya, and M.E. Kost, Hydrides of Samarium-Ruthenium Intermetallides, Russ. J. Lnorg. Chem., 1978, 23, p 3370–3373.
A. Palenzona, The Crystal Structure of the Rare Earth Rich Ruthenium Compounds R3ru and R5Ru2, J. Less-Common Metals, 1979, 66, p P27–P33. https://doi.org/10.1016/0022-5088(79)90234-0
P. Sharifrazi, R.C. Mohanty, and A. Raman, Intermediate Phases in Some Rare Earth Metal-Ruthenium Systems, Z. Metallkd., 1984, 75, p 801–805. https://doi.org/10.1515/ijmr-1984-751011
O. Loebich Jr., and E. Raub, Die Legierungen des Rutheniums mit Gadolinium und Dysprosium und ihre magnetischen Eigenschaften, J. Less-Common Met., 1976, 46, p 7–15. https://doi.org/10.1016/0022-5088(76)90172-7
A. Palenzona, and F. Canepa, The Phase Diagram of the Sm-Ru System, J. Less-Common Metals, 1989, 155, p L31–L33. https://doi.org/10.1016/0022-5088(89)90246-4
H. Okamoto, Ru-Sm (Ruthenium-Samarium), J. Phase Equilibria Diffus., 1991, 12, p 253–254. https://doi.org/10.1007/BF02645731
A.E. Dwight, Factors Controlling the Occurrence of Laves Phases and AB5 Compounds Among Transition Elements, Trans. ASM, 1961, 53, p 479–500.
H. Hall, J.F. Cannon, and D.L. Robertson, The Effect of High Pressure on the Formation of LRu2 and LOs2 (L=Lanthanide) Compounds, J. Less Common Metals, 1972, 29, p 140–146. https://doi.org/10.1016/0022-5088(72)90184-1
N. Selhaoui, and O.J. Kleppa, Standard Enthalpies of Formation of Cerium Alloys, Ce + Me (Me= Ru, Rh, Pd, Ir, Pt) and of Lutetium Alloys, Lu+ Me (Me= Rh, Pd, Ir, Pt) by Hightemperature Calorimetry, Int. J. Mater. Res., 1993, 84, p 744–747. https://doi.org/10.1515/ijmr-1993-841103
A. Dębski, R. Dębski, and W. Gąsior, New Features of Entall Database: Comparison of Experimental and Model Formation Enthalpies, Arch. Metall. Mater., 2014, 59, p 1337–1343. https://doi.org/10.2478/amm-2014-0228
F.R. de Boer, R. Boom, W.C.M. Mattens, A.R. Miedema, and A.K. Niessen, Cohesion in Metals. Transition Metal Alloys. North-Holland, Amsterdam, 1988.
W. Kohn, and L.J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects*, Phys. Rev., 1965, 140, p A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
P. Hohenberg, and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, p B864. https://doi.org/10.1103/PhysRev.136.B864
P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G.L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. De Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A.P. Seitsonen, A. Smogunov, P. Umari, and R.M. Wentzcovitch, QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials, J. Phys. Condens. Matter., 2009. https://doi.org/10.1088/0953-8984/21/39/395502
J.P. Perdew, K. Burke, and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, p 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
C. Colinet, Ab-Initio Calculation of Enthalpies of Formation of Intermetallic Compounds and Enthalpies of Mixing of Solid Solutions, Intermetallics, 2003, 11, p 1095–1102. https://doi.org/10.1016/S0966-9795(03)00147-X
G. Ceder, K. Persson, The Materials Project: A Materials Genome Approach, DOE Data Explor. (2010)
S. Kirklin, J.E. Saal, B. Meredig, A. Thompson, J.W. Doak, M. Aykol, S. Rühl, and C. Wolverton, The Open Quantum Materials Database (OQMD): Assessing the Accuracy of DFT Formation Energies, npj Comput. Mater., 2015, 1, p 15010. https://doi.org/10.1038/npjcompumats.2015.10
C. Oses, E. Gossett, D. Hicks, F. Rose, M.J. Mehl, E. Perim, I. Takeuchi, S. Sanvito, M. Scheffler, Y. Lederer, and O. Levy, Supporting Information: AFLOW-CHULL Manual AFLOW-CHULL: Cloud-Oriented Platform for Autonomous Phase Stability Analysis, J. Chem. Inf. Model., 2018, 58, p 2477–2490. https://doi.org/10.1021/acs.jcim.8b00393
K. Choudhary, K.F. Garrity, A.C.E. Reid, B. Decost, A.J. Biacchi, A.R.H. Walker, Z. Trautt, J. Hattrick-simpers, A.G. Kusne, A. Centrone, A. Davydov, J. Jiang, R. Pachter, G. Cheon, E. Reed, A. Agrawal, X. Qian, V. Sharma, H. Zhuang, and S.V. Kalinin, The Joint Automated Repository for Various Integrated Simulations (JARVIS) for Data-Driven Materials Design, npj Comput. Mater., 2020, 6, p 173. https://doi.org/10.1038/s41524-020-00440-1
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15, p 317–425. https://doi.org/10.1016/0364-5916(91)90030-N
O. Kister, and A.T. Redlich, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40, p 345–348. https://doi.org/10.1021/ie50458a036
M. Hillert, and M. Jarl, A Model for Alloying Effects in Ferromagnetic Metals, Calphad, 1978, 2, p 227–238. https://doi.org/10.1016/0364-5916(78)90011-1
G. Kaptay, The Exponential Excess Gibbs Energy Model Revisited, Calphad Comput. Coupling Phase Diagr. Thermochem., 2017, 56, p 169–184. https://doi.org/10.1016/j.calphad.2017.01.002
Q. Guo, and O.J. Kleppa, Standard Enthalpies of Formation of Neodymium Alloys, Nd + Me ( Me - Ni, Ru, Rh, Pd, Ir, Pt ), by High-Temperature Direct Synthesis Calorimetry, Metall. Mater. Trans. B., 1995, 26B, p 275–279. https://doi.org/10.1007/BF02660969
A.L. Shilov, L.N. Padurets, and M.E. Kost, Formation Enthalpy Determination for Intermetallic Compounds and Their Hydrides from Differential Thermal Analysis Data, Russ. J. Phys. Chem., 1983, 57, p 555–560.
R.F. Zhang, S.H. Sheng, and B.X. Liu, Predicting the Formation Enthalpies of Binary Intermetallic Compounds, Chem. Phys. Lett., 2007, 442, p 511–514. https://doi.org/10.1016/j.cplett.2007.06.031
Q. Guo, and O.J. Kleppa, Standard Enthalpies of Formation of Dysprosium Alloys, Dy + Me (Me ≡ Ni, Ru, Rh, Pd, Ir, and Pt), by High-Temperature Direct Synthesis Calorimetry, Metall. Mater. Trans. B, 1996, 27, p 417–422. https://doi.org/10.1007/BF02914906
N. Selhaoui, J. Charles, O.J. Kleppa, L. Bouirden, and J.C. Gachon, The Ruthenium-Yttrium System: An Experimental Calorimetric Study with a Phase Diagram Optimization, J. Solid State Chem., 1998, 138, p 302–306. https://doi.org/10.1006/jssc.1998.7795
N. Selhaoui, and O.J. Kleppa, Standard Enthalpies of Formation of Lanthanum alloys, La + Me (Me=Ru, Rh, Pd, Os, Ir, Pt), by High-Temperature Calorimetry, J. Alloys Compd., 1993, 191, p 155–158. https://doi.org/10.1016/0925-8388(93)90289-Y
G.F. Voronin, Thermodynamic Properties of Intermediate Phases with Narrow Regions of Homogeneity, J. Russ. J. Phys. Chem., 1976, 50, p 607–611.
J.H. Zhu, C.T. Liu, L.M. Pike, and P.K. Liaw, Enthalpies of Formation of Binary Laves Phases, Intermetallics, 2002, 10, p 579–595. https://doi.org/10.1016/S0966-9795(02)00030-4
N. Selhaoui, J. Charles, L. Bouirden, and J.C. Gachon, Optimization of La-Ru System, Ann. Chim. Sci. Des. Mater., 1999, 24, p 97–104.
S. Kardellass, V.P. Vassiliev, K. Mahdouk, N. Laaroussi, and N. Selhaoui, Excess Thermodynamic Properties of Solutions in Ln-Ru (Ln = Nd, Gd, Dy) Binary Systems Based on Quadratic, Exponential and Combined Models Supported by Ab-Initio Calculations, J. Phase Equilibria Diffus., 2023. https://doi.org/10.1007/s11669-022-01023-x
A. Takeuchi, and A. Inoue, Mixing Enthalpy of Liquid Phase Calculated by Miedema’s Scheme and Approximated with Sub-regular Solution Model for Assessing Forming Ability of Amorphous and Glassy Alloys, Intermetallics, 2010, 18, p 1779–1789. https://doi.org/10.1016/j.intermet.2010.06.003
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kardellass, S., Corvalan-Moya, C. & Vassiliev, V.P. Thermodynamic Modeling of the (Ce and Sm)-Ru Binary Systems Based on Linear, Exponential, and Combined Models Aided by Ab-Initio Calculations. J. Phase Equilib. Diffus. 44, 300–323 (2023). https://doi.org/10.1007/s11669-023-01043-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01043-1