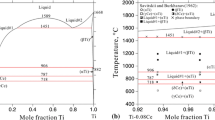
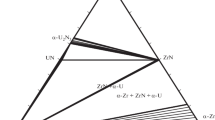
Abstract
The systematic thermodynamic optimization of the Ln-Ru (where Ln = Nd, Gd, and Dy) binary systems is performed using the CALPHAD approach. Using this approach, experimental information published in literature, including both phase equilibria and thermodynamic data, are critically evaluated. The thirteen intermediate phases with stoichiometries, such as Ln3Ru, Nd5Ru2, Ln7Ru3, Nd5Ru3, Ln73Ru27, Ln2Ru, and LnRu2, which have been reported for the three binary Ln-Ru systems are considered as stoichiometric compounds. An exponential and a quadratic model are used to describe the temperature dependence of the excess quantities for the Liquid, (αNd), (βNd), (αDy), (βDy), (αGd), (βGd), and (Ru) solution phases. The results were compared with those from a combined quadratic-exponential temperature dependence description of the excess energies. Using the Thermo-Calc software, self-consistent sets of thermodynamic parameters were obtained to describe the Gibbs energies of the numerous phases in the Ln-Ru binary systems. The calculated results are in good agreement with the available phase equilibria and thermodynamic data.














Similar content being viewed by others
References
J.H. Rademaker, R. Kleijn, and Y. Yang, Recycling as a Strategy Against Rare Earth Element Criticality: A Systemic Evaluation of the Potential Yield of NdFeB Magnet Recycling, Environ. Sci. Technol., 2013, 47, p 10129–10136. https://doi.org/10.1021/es305007w
K.M. Goodenough, J. Schilling, E. Jonsson, P. Kalvig, N. Charles, J. Tuduri, E.A. Deady, M. Sadeghi, H. Schiellerup, A. Müller, G. Bertrand, N. Arvanitidis, D.G. Eliopoulos, R.A. Shaw, K. Thrane, and N. Keulen, Europe’s Rare Earth Element Resource Potential: An Overview of REE Metallogenetic Provinces and Their Geodynamic Setting, Ore Geol. Rev., 2016, 72, p 838–856. https://doi.org/10.1016/j.oregeorev.2015.09.019
Ad hoc Working Group, Report on critical raw materials for the EU, European Commission, 2015.
X. Du, and T.E. Graedel, Science of the Total Environment Uncovering the End Uses of the Rare Earth Elements, Sci. Total Environ., 2013, 462, p 781–784. https://doi.org/10.1016/j.scitotenv.2013.02.099
L. Schlapbach, and A. Züttel, Hydrogen-Storage Materials for Mobile Applications, Nature, 2001, 414, p 353–358. https://doi.org/10.1038/35104634
K. Buschow, S. Jaakkola, S. Parviainen, S. Penttila, and K.H.J. Buschow, Reports on Progress in Physics Intermetallic Compounds of Rare-Earth and 3D Transition Metals Intermetallic Compounds of Rare-Earth and 3D Transition Metals, Rep. Prog. Phys., 1977, 93, p 1179. https://doi.org/10.1088/0034-4885/40/10/002
K.H.J. Buschow, Permanent Magnet Materials Based on Tetragonal Rare Earth Compounds of the Type RFe12-xMx, J. Magn. Magn. Mater., 1991, 100, p 79–89. https://doi.org/10.1016/0304-8853(91)90813-P
K. Binnemans, P.T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton, and M. Buchert, Recycling of Rare Earths: A Critical Review, J. Clean. Prod., 2013, 51, p 1–22. https://doi.org/10.1016/j.jclepro.2012.12.037
N. Saunders, and A.P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide. Pergamon/Elsevier, Oxford, 1998.
L. Kaufman, and H. Bernstein, Computer Calculation of Phase Diagrams (With Special Reference to Refractory Metals). Academic Press Inc, New York, 1970.
H. Lukas, S. Fries, and B. Sundman, Computational Thermodynamics: The Calphad Method. Cambridge University Press, Cambridge, UK, 2007.
G. Kaptay, Materials Equilibria in Macro-, Micro- and Nano-systems, Raszter Ny. (2011).
R. Schmid-Fetzer, D. Andersson, P.Y. Chevalier, L. Eleno, O. Fabrichnaya, U.R. Kattner, B. Sundman, C. Wang, A. Watson, L. Zabdyr, and M. Zinkevich, Assessment Techniques, Database Design and Software Facilities for Thermodynamics and Diffusion, Calphad, 2007, 31, p 38–52. https://doi.org/10.1016/j.calphad.2006.02.007
G. Kaptay, Nano-Calphad: Extension of the Calphad Method to Systems with Nano-phases and Complexions, J. Mater. Sci., 2012, 47, p 8320–8335. https://doi.org/10.1007/s10853-012-6772-9
B. Jansson, Computer Operated Methods for Equilibrium Calculations and Evaluation of Thermochemical Model Parameters. Report, Royal Institute of Technology, Stockholm Sweden, 1984.
B. Sundman, B. Jansson, A. J-O, THE THERMO-CALC DATABANK SYSTEM, Calphad 9 (1985) 153–190. https://doi.org/10.1016/0364-5916(85)90021-5.
S.L. Chen, S. Daniel, F. Zhang, Y.A. Chang, W.A. Oates, and R. Schmid-Fetzer, On the Calculation of Multicomponent Stable Phase Diagrams, J. Phase Equilibria, 2001, 22, p 373–378. https://doi.org/10.1361/105497101770332910
G. Kaptay, A New Equation for the Temperature Dependence of the Excess Gibbs Energy of Solution Phases, Calphad, 2004, 28, p 115–124. https://doi.org/10.1016/j.calphad.2004.08.005
T. Abe, K. Ogawa, and K. Hashimoto, Analysis of Miscibility Gaps Based on the Redlich–Kister Polynomial for Binary Solutions, Calphad, 2012, 38, p 161–167. https://doi.org/10.1016/j.calphad.2012.06.006
G. Kaptay, On the Tendency of Solutions to Tend Toward Ideal Solutions at High Temperatures, Metall. Mater. Trans. A Phys. Metall. Mater. Sci., 2012, 43, p 531–543. https://doi.org/10.1007/s11661-011-0902-x
G. Kaptay, On the Abilities and Limitations of the Linear, Exponential and Combined Models to Describe the Temperature Dependence of the Excess Gibbs Energy of Solutions, Calphad, 2014, 44, p 81–94. https://doi.org/10.1016/j.calphad.2013.08.007
J.F. Elliott, and C.H.P. Lupis, Correlation Between Excess Entropy and Enthalpy Functions, Trans. Met. Soc. AIME, 1966, 236, p 130.
A. Palenzona, and F. Canepa, The Phase Diagrams of the La-Ru and Nd-Ru Systems, J. Less-Common Met., 1990, 157, p 307–313. https://doi.org/10.1016/0022-5088(90)90186-N
P. Sharifrazi, R.C. Mohanty, and A. Raman, Intermediate Phases in Some Rare Earth Metal-Iridium Systems, Z. Metallkd., 1984, 75, p 801–805. https://doi.org/10.1515/ijmr-1989-800309
H. Okamoto, Nd-Ru (Neodymium-Ruthenium), J. Phase Equilibria, 1991, 12, p 250–252. https://doi.org/10.1007/BF02645729
O. Loebich Jr., and E. Raub, Die Legierungen des Rutheniums mit Gadolinium und Dysprosium und ihre Magnetischen Eigenschaften, J. Less-Common Met., 1976, 46, p 7–15. https://doi.org/10.1016/0022-5088(76)90172-7
K.H. Hellwege, Strukturdaten der Elemente und intermetallischen Phasen Landolt-Börnstein, New Series, Group 3, Vol. 6. Springer, Berlin, 1971.
R.M. Bozorth, B.T. Matthias, H. Suhl, E. Corenzwit, and D.D. Davis, Magnetization of Compounds of Rare Earths with Platinum Metals, Phys. Rev., 1959, 115, p 1595–1596. https://doi.org/10.1103/PhysRev.115.1595
O. Loebich Jr., and E. Raub, Magnetic Properties of Alloys of Rh with Lanthanides (in German), Magn. Prop. Alloy Rh Lanthanides., 1975, 10, p 1017–1022.
O. Loebich Jr., and E. Raub, Das magnetische Verhalten der Legierungen des Palladiums Mit Gadolinium, Dysprosium und Holmium, J. Less-Common Met., 1973, 31, p 111–118. https://doi.org/10.1016/0022-5088(73)90134-3
A.E. Berkowitz, F. Holtzberg, and S. Methfessel, New Ferromagnetic 5:2 Compounds in the Rare-Earth—Palladium Systems, J. Appl. Phys., 1964, 35, p 1030–1031. https://doi.org/10.1063/1.1713364
R. Ferro et al., Bericht der Eur. Atomgemeinschaft, EUR-1894i. (1964).
R.D. Hutchens, V.U.S. Rao, J.E. Greedan, W.E. Wallace, and R.S. Craig, Magnetic and Electrical Characteristics of REPd3 Intermetallic Compounds, J. Appl. Phys., 1971, 42, p 1293–1294. https://doi.org/10.1063/1.1660221
W.E. Gardner, J. Penfold, T.F. Smith, and I.R. Harris, The Magnetic Properties of Rare Earth-Pd3 Phases, J. Phys. F Met. Phys., 1972, 2, p 133–150. https://doi.org/10.1088/0305-4608/2/1/019
J. Pierre, and E. Siaud, Structure Cristalline et Propriétés Magnétiques du Composé GdPd, Compt. Rend. Acad. Sci., 1968, 266, p 1483–1485.
Q. Guo, and O.J. Kleppa, Standard Enthalpies of Formation of Neodymium Alloys, Nd + Me (Me-Ni, Ru, Rh, Pd, Ir, Pt), by High-Temperature Direct Synthesis Calorimetry, Metall. Mater. Trans. B., 1995, 26B, p 275–279. https://doi.org/10.1007/BF02660969
A.L. Shilov, L.N. Padurets, and M.E. Kost, Formation Enthalpy Determination for Intermetallic Compounds and Their Hydrides from Differential Thermal Analysis Data, Russ. J. Phys. Chem., 1983, 57, p 555–560.
N. Selhaoui, J. Charles, L. Bouirden, and J.C. Gachon, Optimization of La-Ru System, Ann. Chim. Sci. Des Mater., 1999, 24, p 97–104.
A. Debski, R. Debski, and W. Gasior, New Features of Entall Database: Comparison of Experimental and Model Formation Enthalpies, Arch. Metall. Mater., 2014, 59, p 1337–1343. https://doi.org/10.2478/amm-2014-0228
F.R. de Boer, R. Boom, W.C.M. Mattens, A.R. Miedema, and A.K. Niessen, Cohesion in metals. Transition metal alloys. North-Holland, Amsterdam, 1988.
A. Jain, S.P. Ong, G. Hautier, W. Chen, W.D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder, and K.A. Persson, Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation, APL Mater., 2013, 1, p 011002. https://doi.org/10.1063/1.4812323
S. Kirklin, J.E. Saal, B. Meredig, A. Thompson, J.W. Doak, M. Aykol, S. Rühl, and C. Wolverton, The Open Quantum Materials Database (OQMD): Assessing the Accuracy of DFT Formation Energies, npj Comput. Mater., 2015. https://doi.org/10.1038/npjcompumats.2015.10
R.F. Zhang, S.H. Sheng, and B.X. Liu, Predicting the Formation Enthalpies of Binary Intermetallic Compounds, Chem. Phys. Lett., 2007, 442, p 511–514. https://doi.org/10.1016/j.cplett.2007.06.031
N. Selhaoui, and O.J. Kleppa, Standard Enthalpies of Formation of Lanthanum Alloys, La + Me (Me = Ru, Rh, Pd, Os, Ir, Pt), by High-Temperature Calorimetry, J. Alloys Compd., 1993, 191, p 155–158. https://doi.org/10.1016/0925-8388(93)90289-Y
C. Oses, E. Gossett, D. Hicks, F. Rose, M.J. Mehl, E. Perim, I. Takeuchi, S. Sanvito, M. Scheffler, Y. Lederer, and O. Levy, Supporting Information: AFLOW-CHULL Manual AFLOW-CHULL: Cloud-Oriented Platform for Autonomous Phase Stability Analysis, J. Chem. Inf. Model., 2018, 58, p 2477–2490. https://doi.org/10.1021/acs.jcim.8b00393
Q. Guo, and O.J. Kleppa, Standard Enthalpies of Formation of Dysprosium Alloys, Dy + Me (Me ≡ Ni, Ru, Rh, Pd, Ir, and Pt), by High-Temperature Direct Synthesis Calorimetry, Metall. Mater. Trans., 1996, 27B, p 417–422. https://doi.org/10.1007/BF02914906
K. Choudhary, K.F. Garrity, A.C.E. Reid, B. Decost, A.J. Biacchi, A.R.H. Walker, Z. Trautt, J. Hattrick-simpers, A.G. Kusne, A. Centrone, A. Davydov, J. Jiang, R. Pachter, G. Cheon, E. Reed, A. Agrawal, X. Qian, V. Sharma, H. Zhuang, and S.V. Kalinin, The Joint Automated Repository for Various Integrated Simulations (JARVIS ) for Data-Driven Materials Design, Npj Comput. Mater., 2020, 6, p 1–13. https://doi.org/10.1038/s41524-020-00440-1
A. Takeuchi, and A. Inoue, Mixing Enthalpy of Liquid Phase Calculated by Miedema’s Scheme and Approximated with Sub-regular Solution Model for Assessing Forming Ability of Amorphous and Glassy Alloys, Intermetallics, 2010, 18, p 1779–1789. https://doi.org/10.1016/j.intermet.2010.06.003
W. Kohn, and L.J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, p A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
P. Hohenberg, and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, p B864. https://doi.org/10.1103/PhysRev.136.B864
P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G.L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. De Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A.P. Seitsonen, A. Smogunov, P. Umari, and R.M. Wentzcovitch, QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials, J. Phys. Condens. Matter., 2009. https://doi.org/10.1088/0953-8984/21/39/395502
J.P. Perdew, K. Burke, and M. Ernzerhof, Generalized Gradient Approximation made Simple, Phys. Rev. Lett., 1996, 77, p 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
C. Colinet, Ab-Initio Calculation of Enthalpies of Formation of Intermetallic Compounds and Enthalpies of Mixing of Solid Solutions, Intermetallics, 2003, 11, p 1095–1102. https://doi.org/10.1016/S0966-9795(03)00147-X
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15, p 317–425. https://doi.org/10.1016/0364-5916(91)90030-N
M. Hillert, and M. Jarl, A Model for Alloying Effects in Ferromagnetic Metals, Calphad, 1978, 2, p 227–238. https://doi.org/10.1016/0364-5916(78)90011-1
O. Kister, and A.T. Redlich, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40, p 345–348. https://doi.org/10.1021/ie50458a036
G. Kaptay, The Exponential Excess Gibbs Energy Model Revisited, Calphad, 2017, 56, p 169–184. https://doi.org/10.1016/j.calphad.2017.01.002
N. Selhaoui, and O.J. Kleppa, Standard Enthalpies of Formation of Cerium Alloys, Ce + Me (Me= Ru, Rh, Pd, Ir, Pt) and of Lutetium Alloys, Lu+ Me (Me= Rh, Pd, Ir, Pt) by Hightemperature Calorimetry, Z. Metallkd., 1993, 84, p 744–747. https://doi.org/10.1515/ijmr-1993-841103
A. Palenzona, and F. Canepa, The Phase Diagram of the Sm-Ru System, J. Less-Common Met., 1989, 155, p L31–L33. https://doi.org/10.1016/0022-5088(89)90246-4
N. Selhaoui, J. Charles, O.J. Kleppa, L. Bouirden, and J.C. Gachon, The Ruthenium-Yttrium System: An Experimental Calorimetric Study with a Phase Diagram Optimization, J. Solid State Chem., 1998, 138, p 302–306. https://doi.org/10.1006/jssc.1998.7795
G.F. Voronin, Thermodynamic Properties of Intermediate Phases with Narrow Regions of Homogeneity, J. Russ. J. Phys. Chem., 1976, 50, p 607–611.
B. Predel, Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys, Landolt-Börnstein, New Series, Vol. 5, 1st edn. Springer, Berlin, 1993.
T.B. Massalski, H. Okamoto, P. Subramanian, L. Kacprzak, and W.W. Scott, Binary Alloy Phase Diagrams. ASM International, Materials Park, 1986. https://doi.org/10.31399/asm.hb.v03.a0006247
H. Okamoto, Ru-Sm (Ruthenium-Samarium), J. Phase Equilibria Diffus., 1991, 12, p 253–254. https://doi.org/10.1007/BF02645731
V.P. Vassiliev, A. Benaissa, and A.F. Taldrik, Thermodynamics Analysis of the Rare Earth Metals and Their Alloys with Indium in Solid State, J. Alloys Compd., 2013, 572, p 118–123. https://doi.org/10.1016/j.jallcom.2013.03.063
V.P. Vassiliev, and V.A. Lysenko, A New Approach for the Study of Thermodynamic Properties of Lanthanide Compounds, Electrochim. Acta., 2016, 222, p 1770–1777. https://doi.org/10.1016/j.electacta.2016.11.075
V.P. Vassiliev, V.A. Lysenko, and M. Gaune-Escard, Relationship of Thermodynamic Data with Periodic Law, Pure Appl. Chem., 2019, 91, p 879–893. https://doi.org/10.1515/pac-2018-0717
V.N. Eremenko, V.G. Khorujaya, P.S. Martsenyuk, and K.Y. Korniyenko, The Scandium-Ruthenium Phase Diagram, J. Alloys Compd., 1995, 217, p 213–217. https://doi.org/10.1016/0925-8388(94)01321-7
H. Okamoto, Ru-Sc (Ruthenium-Scandium), J. Phase Equilibria Diffus., 2008, 29, p 387–388. https://doi.org/10.1007/s11669-008-9343-7
A. Palenzona, The Phase Diagram of the Ce-Ru System, J. Alloys Compd., 1991, 176, p 241–246. https://doi.org/10.1016/0925-8388(91)90031-P
N. Selhaoui, J. Charles, L. Bouirden, and J.C. Gachon, Optimization of the Binary Ce–Ru System, J. Alloys Compd., 1998, 269, p 166–172. https://doi.org/10.1016/S0925-8388(98)00125-X
Acknowledgments
We acknowledge Professor Lorie Wood from the University of Colorado (USA) for the language help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kardellass, S., Vassiliev, V.P., Mahdouk, K. et al. Excess Thermodynamic Properties of Solutions in Ln-Ru (Ln = Nd, Gd, Dy) Binary Systems Based on Quadratic, Exponential and Combined Models Supported by Ab-Initio Calculations. J. Phase Equilib. Diffus. 44, 43–75 (2023). https://doi.org/10.1007/s11669-022-01023-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-022-01023-x