Abstract
Phase equilibria in the Fe-Mn-Zr system have been studied experimentally using SEM/EDX methods, EBSD and powder x-ray diffraction. Quasi binary section of ZrFe2-ZrMn2 has been constructed. Invariant reactions were studied using differential thermal analysis followed by SEM/EDX. Using obtained experimental results at 1073 K, ternary isothermal section has been constructed. A self-consistent thermodynamic description of the Fe-Mn-Zr system has been developed using the CALPHAD method based on the new experimental information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laves phases have gained much attention in the last 20 years due to their high strength at high temperatures, high melting points, low density, excellent hardness as well as their notable corrosion and oxidation resistance.[1,2] Materials based on the Fe-Mn-Zr system are very promising for many structural applications[2] as well as for hydrogen storage.[3]
Moreover, the Fe-Mn-Zr system is of great interest for manufacturing of the TRIP-Matrix-Composite material based on high-alloyed austenitic stainless TRIP-steel (Transformation Induced Plasticity) reinforced by ceramic particles of Mg-PSZ (magnesia partially stabilized zirconia).[4] Such composite materials have a wide range of technological applications especially in automotive industry due to excellent mechanical properties such as ductility, plasticity, high strength, and energy absorption during compression.[4,5] Development of such composite materials requires the knowledge of thermodynamic properties and phase relations. Apparently, phase relations in the Fe-Mn-Zr system have become subject of great interest and the detailed knowledge of its thermodynamic properties is essential for the modern industry of composite materials and structural steels. Therefore, the main purpose of this work is experimental investigation of the phase relations in the Fe-Mn-Zr system and its thermodynamic modelling.
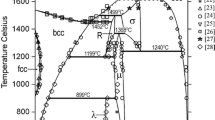
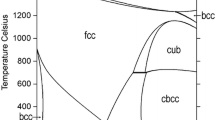
According to literature data, phase diagrams of binary sub-systems of the ternary Fe-Mn-Zr system are well known. The last experimental investigation and thermodynamic modelling of the Fe-Zr and Mn-Zr systems have been performed previously.[6,7,8] The latest version of phase diagram Fe-Mn was determined by Witusiewicz et al.[9] Experimental data used by Witusiewicz et al.[9] include data previously used by Huang[10] and Lee et al.[11], supplemented by more recent experimental results from Li et al.[12] and data from the work of Witusiewicz et al.[13] on heat capacity, enthalpy of transformation and magnetic properties of present solid solutions. Nevertheless, this new re-evaluation of the thermodynamic parameters of the system practically did not change the phase diagram and it is almost identical to the one calculated by Huang.[10] In the work of Witusiewicz et al.[9] the re-assessment of the Fe-Mn system was done also considering metastable phases, which are described by scattered data available in the literature. In the present work, metastable phases were not taken into account, therefore the thermodynamic assessment of Huang[10] was accepted. All stable intermetallic phases and their crystal structures are listed in Table 1.
Experimental investigations of phase relations in the Fe-Mn-Zr system were focused on a part of the diagram representing a vertical section of ZrMn2-ZrFe2.[19,20] However, available data is contradictory and quite scarce. Based on the results of Pet’kov[20] only solid solutions of the C14 and C15 Laves phases were proposed with solubility ranges limited at 0.6 and 0.8 mole % of C15-ZrFe2. On the other hand, based on the magnetic investigations,[21] it was stated that in this composition range the phase with C36 structure (MgNi2 type) was formed. The entire ternary Fe-Mn-Zr system has never been investigated.
2 Materials and Methods
2.1 Sample Preparation and Characterization
Samples for study of phase relations in the Fe-Mn-Zr system were prepared from slugs of Zr (99.5%, Alfa Aesar), pieces of Fe (99.99%, Alfa Aesar) and Mn (99.95%, Alfa Aesar) by arc-melting in an argon (99.999%) atmosphere. To achieve good compositional homogeneity, the samples were turned over and re-melted at least three times. The melting chamber was evacuated to a pressure of ~ 10−5 atm and backfilled with argon 3 times. An ingot of pure Ti (ca. 3 g) was placed in one of the molds in the melting chamber and was melted before the melting of the samples to adsorb residual oxygen and reduce possible oxidation. The weight loss after melting was determined to be less than 2 wt.%. Afterwards, samples obtained by arc melting were encapsulated in quartz tubes under Ar atmosphere. The pressure of Ar inside the tubes was chosen to reach a pressure of 1 atm. at annealing temperature. The samples were homogenized for 7 days at 1073 K.
For the x-ray powder diffraction (XRD), the samples were pulverized in an agate mortar. The measurements were performed on an URD63 diffractometer (Seifert, FPM, Freiberg) with Bragg–Brentano geometry. The device is equipped with a graphite monochromator using Cu-Kα (λ = 1.5418 Å) radiation in a 2θ-range from 15 to 110°. The goniometer of the diffractometer has the Bragg–Brentano geometry. Rietveld refinement was performed using MAUD software[22] for quantitative analysis of measured diffraction patterns. Based on the results of the Rietveld refinement, volume fractions of phases present as well as their lattice parameters were obtained.
Microstructural investigations have been performed by scanning electron microscopy combined with energy dispersive x-ray spectrometry (SEM/EDX) using LEO 1530 Gemini (Zeiss, Germany) microscope. Chemical compositions of the samples as well as of phases present were measured using an EDX detector (Bruker AXS Microanalysis GmbH) with an accuracy of ± 4 at.%. Composition of the phases was measured in at least three different points and the bulk composition of the sample was measured on the whole area of the sample slice. All the samples were polished down to 1 µm and an alcohol-based lubricant was used to exclude oxygen contamination. Samples from the SEM/EDX investigation were used for EBSD as well.
The electron backscatter diffraction (EBSD) scans were carried out using a JEOL JSM 7800F scanning electron microscope (SEM) with an acceleration voltage of 20 keV and beam current of approx. 10 nA. The Backscattered Kikuchi Diffraction (BKD) patterns are acquired using the EDAX TEAM software and analyzed using the TSL OIM Analyses 8. The acquired BKD patterns have been treated using high and low pass filtering, as well as an adjustment of the grey scale histogram to maximize the features visible and distinguishable.[23]
2.2 Thermoanalysis
To determine temperatures of invariant reactions, differential thermal analysis (DTA) was performed using DTA SETSYS Evolution 1750 (SETARAM, Caluire, France) at a heating rate 10 K/min under helium (99.999%) atmosphere flow of 20 ml/min. Alumina crucibles were lined with a bed of yttria to prevent reaction between zirconium and alumina. Crucibles were also covered with a lid to avoid evaporation of highly volatile manganese. For more accurate temperature measurement, temperature calibration was done using melting points of Al (99.999%), Au (99.99%) and Ni (99.997%). Sample microstructures after DTA have been investigated using SEM/EDX as well.
3 Modelling
The Thermo-Calc software was applied for the optimization of thermodynamic parameters based on the CALPHAD approach[24] and for phase diagram calculations using Parrot and POLY-3 modules, resepctively. Individual weights were given to every type of experimental data considering possible deviation and accuracy of each experimental method.[25] Thermodynamic descriptions of binary sub-systems Zr-Fe, Zr-Mn and Fe-Mn were accepted from the works of Saenko et al.,[6] Kuprava et al.[8] and Huang,[10] respectively.
The thermodynamic description of the Gibbs energy of each a pure element \(i (i=\mathrm{Fe}, \mathrm{Zr},\mathrm{Mn})\) in the \(\Phi \) phase referred to the enthalpy of the stable phase at 298.15 K was accepted from SGTE Unary database version 5.0[26] in the following form:
The magnetic contribution to the thermodynamic properties of the elements was taken into account according to the Inden–Hillert–Jarl[27] formalism (Eq 2).
where \(\tau \) = T/T*, T* being the critical temperature (the Curie temperature TC for ferromagnetic materials or the Neel temperature TN for antiferromagnetic materials), \({\beta }_{0}\) the average magnetic moment per atom and \(g(\tau )\) is a function depending on \(\tau \).[26]
Substitutional solutions were modelled using following equation:
where \({G}_{m}^{\Phi ,srf}=\sum_{i}^{n}{x}_{i}^{0}{G}_{i}^{\Phi }\) is the surface of reference term, \({G}_{m}^{\Phi ,conf}=RT\sum_{i}^{n}{x}_{i}ln{x}_{i}\) is the Gibbs free energy resulting from the configurational entropy of mixing for a disordered solution, and \({G}_{m}^{\Phi ,Ex}\) is the excess Gibbs free energy of mixing.
The excess Gibbs free energy of binary mixing was modelled using Redlich–Kister polynomials,[28] expressed as:
where \(^{\upsilon } L_{i,j}^{{\Phi }} \) are binary interaction parameters with n ranging from 0 to n.
The model for ternary system contains sum of binary interactions and ternary interactions described by the Redlich–Kister–Muggianu polynomials and can be expressed as:
where \({L}_{ijk}^{i}\) , \({L}_{ijk}^{j}\) and \({L}_{ijk}^{k}\) are ternary interaction parameters.
In our study, ternary interaction parameters were used only to describe mixing in the liquid phase.
The sublattice model in the form of the compound energy formalism was used for solid phases which have several crystallographic sites. If solid solutions and compounds have two or more different sites in the crystal structure that can be occupied by atoms, ions or more complex species, their homogeneity ranges can be described by the compound energy formalism (CEF) developed by Hillert.[29] The structure of the phase can be represented by the formula (A,B)k(C,D)m, indicating that A and B species mix on the first sublattice, while C and D mix on the second sublattice. Coefficients k and m are stoichiometric numbers for the first and second sublattice, respectively. The Gibbs energy in CEF is given as
where \({y}_{i}^{(1)}\) and \({y}_{j}^{(2)}\) represent the mole fraction of constituents i and j on the first and second sublattices, respectively; \({G}_{ij}\) is the Gibbs energy of an end-member compound of the solution, in which the first sublattice is occupied only by the constituent i and the second only by the constituent j; T is the temperature in K; \({G}^{E}\) is the excess Gibbs energy; and \({S}^{C}\) is the configurational entropy which is defined as following
where n and m are the stoichiometric numbers of each sublattice and R is the ideal gas constant.
The excess Gibbs energy is expressed using Redlich-Kister equations as
where the parameter \({L}_{i,k:j}^{(1)}\) expresses interactions between species i and k on the sublattice (1) when the sublattice (2) is occupied by j, while \({L}_{i:j,m}^{(2)}\) is interaction parameter between species j and m on the sublattice (2) when the sublattice (1) is occupied by i.
The Gibbs energy of reciprocal reaction between end-members ∆G is usually assumed to be equal to zero. Therefore, if A and B are mixing on the first sublattice, C and D are mixing on the second sublattice, the Gibbs energy of one end-member can be determined from reciprocal reaction if they are known for other three end-members and the Gibbs energy of reciprocal reaction \(\Delta^{0} G^{rec}\) is assumed to be equal to zero.
Homogeneity ranges of phases BCC (β-Zr, α,δ-Fe, δ-Mn solid solution), FCC (γ-Fe, γ-Mn based solid solution ), HCP (α-Zr based solid solution), CUB (β-Mn solid solution) and CBCC (α-Mn based solid solution), as well as the liquid phase were described by the substitutional model with one sublattice in the form of (Zr,Fe,Mn). Intermetallic compounds with homogeneity range were described using compound energy formalism.[29]
4 Results and Discussion
A preliminary thermodynamic database of Zr-Fe,[6] Zr-Mn[8] and Fe-Mn[10] was compiled based on binary extrapolations into the ternary without the inclusion of ternary mixing parameters. Several compositions in the Fe-Mn-Zr system were then selected for experimental investigation based on the preliminary calculations. Samples’ nominal and measured compositions, annealing temperature and duration, SEM/EDX and XRD results are presented in Table 2. Due to fast kinetics of the polymorphous transformation of β-Zr into α-Zr, ex-situ XRD measurements indicate the presence of the α-Zr phase despite that β-Zr is the equilibrium phase at 1073 K. Due to this fact and based on composition measurements, the Zr-rich phase is identified as β-Zr. In samples #5 and #7 the phases that are presumably observed with SEM were not found by XRD and thus the volume fraction is not given for them. Although, the binary Fe-Zr phase diagram indicated the occurrence of tetragonal Zr2Fe phase, there have been reports about the cubic variation of the phase,[30,31] which was observed in the sample #6. Possibly the cubic structure is metastable. Sample #1 was only used for a DTA experiment without prior annealing, after which it was only analyzed by means of SEM/EDX.
4.1 Phase Equilibria
Phase relations in the quasi-binary ZrMn2-ZrFe2 section play a significant role in phase equilibria in the whole ternary system and thus were collected from all samples and discussed along with the ternary phase equilibria. Because of difficulties to distinguish between C14 and C36-structured Laves-phases using XRD method, an arbitrary EBSD investigation was carried out to identify present Laves-phases in the quasi-binary section.
BKD patterns of the measured data and the dynamically simulated[32] C14, C15 and C36 structure showing the same orientation and pattern center. One of the main bands for distinguishing the structures is marked in Fig. 1 exemplary with red arrows \(\left(\overline{2 }021\right)\) in C14 notation. This band is absent in the C15 structure; hence measured sample is not C15-structured. The blue arrows indicate features in the simulated structures, which are absent in the measured data and should be visible due to the pattern quality achieved. Distinguishing between the C14 and C36 structure is difficult, since the major differences in the BKD patterns for the shown orientation lay in bands which are broad (due to low lattice spacings) and are of minor intensity (due to low structure factors).
The measured data obtained from the samples #2 (Fig. 1), #5, #6 and #8 are clearly best fitted (via a cross correlation of the measured BKD pattern with the simulated ones) by the C14 structure, if the average fit qualities (C14:64%, C36:62% and C15:33%) are considered. The best fit with the C14 structure supports the observation made by comparing the features of the BKD patterns, that observed structure is indeed C14 and not C36.
Composition of the sample #6 (Fig. 2) is very close to the ZrMn2-ZrFe2 quasi-binary section of the ternary system. By means of XRD it was revealed that sample consists mostly of C14-ZrMn2 with just 6 vol.% of cubic Zr2Fe, which means that the sample composition lies in a two-phase region very close to the boundary of C14-ZrMn2 single-phase region. Composition of brighter and darker formations in the microstructure (Fig. 2) vary up to 4 at.% Mn, although both represent the C14-ZrMn2 phase. These inhomogeneities could be explained by non-equilibrium crystallization. Homogenization of the microstructures during annealing appeared was sluggish due to the high thermal stability of the C14 Laves-phase. White phase in microstructure corresponds to the Zr2Fe composition.
The microstructure of the sample #8 is shown in Fig. 3. The white matrix phase is β-Zr with 98.3 at.% Zr content. The darker inclusions are observed to vary in manganese content up to 15 at.%, which would suggest presence of another phase. However, XRD indicated only the C14-ZrMn2 and β-Zr phase. To verify absence of another phase, namely C36 Laves-phase proposed in other work,[21] EBSD investigation was performed, which showed presence of only the C14 Laves phase in the sample. Thus, the 15 at.% composition shift could be caused by the inhomogeneities after sample preparation and sluggish kinetics at the annealing temperature.
Both alloys #2 and #5 are in the same three-phase C14-ZrMn2-β-Zr-Zr2Fe region. Figure 4 shows the microstructure of #2 with three distinct phases after the heat treatment. The light colored phase that forms the matrix of the microstructure is β-Zr with 96.7 at.% Zr. A bar-like phase is identified as C14-ZrMn2. The composition of the bar-like constituent lies in the C14-ZrMn2 single-phase region of the quasi-binary section according to the diagram from other work.[20] The grey phase corresponds to the Zr2Fe phase. The Zr2Fe phase composition (Table 2) indicates that Fe atoms are partially substituted by Mn atoms, which is reflected in the thermodynamic description of the phase. In the Fig. 5 showing sample #5, the darkest irregular grains are the C14-ZrMn2, a light colored phase is β-Zr, and the light grey phase is the Zr2Fe. Phase constitution for alloys #5 and #2 is proven to agree with SEM observations by means of XRD. Although, besides the three phases in the sample #5, the microstructure features another constituent that appears slightly darker than Zr2Fe and possesses 53/29.4/17.6 at.% Zr/Fe/Mn composition that is not listed in Table 2. This composition does not match any of the stable binary phases in the constituting sub-systems and does not appear in any other samples. Because of that, it is assumed to be a metastable oxygen-stabilized ZrMn phase observed in a previous work on the Zr-Mn system[8] assuming that Mn was substantially substituted by Fe.
Microstructures of the samples #4 and #7 correspond to the same two-phase field C14-ZrMn2 + γ-(Fe,Mn). However, XRD results indicate presence of mainly C14-ZrMn2 phase and 4 vol.% of γ-(Fe,Mn) in the sample #4, presence of any other phase besides C14-ZrMn2 in the sample #7 was below XRD sensitivity. Composition variation that can be observed on both microstructures belonging to C14-ZrMn2. The lightest colored regions corresponded to the C14 phase with a content of ~16 at.% Mn and up to ~21 at.% Mn in darker regions. The microstructure of sample #4 (Fig. 6) clearly shows presence of two phases which agrees with XRD measurements. It should be noted that C14-ZrMn2 and γ-(Fe,Mn) phases are present in microstructure of sample #7 (Fig. 7), but the measured composition of γ-(Fe,Mn) in the sample #7 was unreliable due to the small size of the γ-(Fe,Mn) grains and was thus not considered in thermodynamic assessment.
Sample #3 (Fig. 8) allowed only a limited phase equilibria study. Due to fast kinetics of decomposition of Zr2Fe into C15-ZrFe2 and Zr3Fe, the microstructure of the sample was affected by decomposition and does not correspond to the equilibrium state at the annealing temperature. Difficulties with quenching of the Zr2Fe phase have already been reported in the work of Saenko et al.[6]. Between the C15-ZrFe2, Zr3Fe and β-Zr grains several new smaller grains of the C15-ZrFe2 can be seen. Their formation, as well as growth of the Zr3Fe phase, could be explained by decomposition of Zr2Fe, which disappeared fully during quenching.
4.2 DTA
A DTA experiment was carried out with the samples #1 and #6 for obtaining data on the phase equilibria in ZrMn2-ZrFe2 section. The DTA heating and cooling curves for the sample #1 are shown in Fig. 9. One distinguishable heat effect is observed at 1901 K on heating. The DTA curves for the sample #6 are presented in Fig. 10. A heat effect in that sample is observed at 1846 K. Heating and cooling curves (Fig. 9 and 10) feature only one peak. The fact that heat effects on cooling occur at higher temperatures means that reactions on cooling and heating do not correspond to each other and represent solidus or liquidus lines. Absence of other distinguishable heat effects in both samples leads to a conclusion that both samples are in single-phase regions of C14 and C15 below melting, respectively, while two-phase regions of C14+L and C15+L are very narrow and lie in between measured samples.
For the determination of an invariant reaction in ternary region DTA experiments were carried out on samples #2 and #5. DTA heating and cooling curves for the sample #2 are shown in Fig. 11 with heat effects at 1190 and 1207 K. There are two eutectic reactions in the constituent systems Zr-Mn and Zr-Fe, that occur 1201 and 1405 K, which lead to the conclusion that the heat effect at 1190 K corresponds to an invariant reaction in the ternary system. The microstructure of the sample #2 presented in Fig. 12 shows a eutectic microstructure (E1) consisting of C14-ZrMn2, β-Zr and Zr2Fe and the bulk eutectic composition is 73/21.5/5.5 at.% Zr/Fe/Mn. Absence of the eutectic heat effect in the sample #5 could be since the sample composition is too far from the eutectic composition. It also means that the β-Zr content is low and thus a three-phase interaction is significantly reduced. The peak observed at 1207 K in the sample 2# (Fig. 11) corresponds to the binary eutectic reaction in Zr-Fe system: \(Liquid \, \leftrightarrow {\upbeta } - {\text{Zr}} + {\text{ Zr}}_{2} {\text{Fe}}\;{12}0{1}\; {\text{K}}\)[15] Its product can be observed in Fig. 12 as finely dotted constituent with measured composition 73.3/24.6/2.1 at.% Zr/Fe/Mn marked in the Figure as e2. The peak at 1206 K in the sample #5 (Fig. 13) corresponds to the same reaction and similar dotted phase can be observed in the microstructure (Fig. 14). Heat effect at 1013 K on cooling in the sample #5 may correspond to the decomposition reaction of Zr2Fe with an undercooling from 1053 K.[6]
Obtained experimental results combined with the data of the binary systems allow partial construction of the isothermal section at 1073 K of the Fe-Mn-Zr system. Three two-phase and one three-phase field are determined, namely C14 + β-Zr, C14 + γ-(Fe,Mn), C15 + C14 and C14 + Zr2Fe + β-Zr. The C15 + C14 region is not marked by a solid line as its boundaries were determined from the samples outside the region. Other parts of the diagram were adjusted using thermodynamic modelling results. Homogeneity region of β-Zr phase was constructed based on the binary systems due to insufficient accuracy of SEM measurements relative to the region extent. Experimental results show that the homogeneity range of the Zr2Fe phase has a 6 at.% Mn extension into the ternary region. DTA investigation revealed a ternary eutectic reaction at 1190 K and composition of 73/21.5/5.5 at.% Zr/Fe/Mn. The width of the quasi-binary section was evaluated from the measurements of the C14-ZrMn2 phase in all samples. EBSD analysis confirmed absence of a C36 structure in the quasi-binary system. The constructed isothermal section of the system at 1073 K is presented in Fig. 15. Invariant equilibria with liquid phase are presented in the Table 3.
4.3 Thermodynamic Modelling
The thermodynamic database of the Fe-Mn-Zr system was derived based on the phase equilibria results obtained in the current work using CALPHAD approach. The optimization procedure was carried out using Parrot module of the Thermo-Calc software.[24]
In the first step, the thermodynamic assessments of the binary sub-systems were collected and combined. Thereafter, necessary constituents were added to the sublattices to describe ternary solubilities according to the obtained results. Then, the Gibbs energies of metastable end-members were manually tuned to best fit the isothermal section. For better fit with the experimental data, mixing parameters were introduced to C14 and C15 phase descriptions. Finally, parameters for the liquid phase were optimized step-by-step to reproduce the experimental data using Parrot.
Additional Mn constituent was added to Fe on the second sublattice in the Zr2Fe description. No mixing parameters were used for this phase.
Despite the observed extension of the γ-(Fe,Mn) phase into the ternary region, this behavior was not modelled, due to scarce of experimental data and unsatisfactory results with one mixing parameter.
C14 and C15 phases are modelled with all the components on both sublattices. Data for end-members were then complemented by the DFT-calculated formation enthalpies of theoretical compounds.[33,34] To describe the two-phase region and width of the homogeneity ranges of Laves phases, a free correction parameter was added to the descriptions of the theoretical compounds (C14-ZrFe2 and C15-ZrMn2). One mixing parameter was added to the C15 description and one to C14. Several end-members of the ZrFe2 and ZrMn2 phases were assessed to reproduce the width and form of the two-phase area C14 + C15 evaluated from the experimental results. For the additional necessary end-members start values were chosen following approaches used in several works[35,36,37,38] dedicated to systems with similar quasi-binary section. Descriptions of metastable C14-ZrFe2 and C15-ZrMn2 end-members were taken from the stable phase descriptions. Slight bend of the C14 homogeneity region towards Zr-rich corner (Fig. 15) was not modelled with the present parameters.
The thermodynamic parameters obtained for the Fe-Mn-Zr system are listed in the Table 4 in Appendix. The calculated isothermal section at 1073 K is presented in Fig. 16, quasi-binary ZrMn2-ZrFe2 section is presented in the Fig. 17, liquidus surface is presented in the Fig. 18.
As it can be seen on Fig. 18, melting points of the Laves phases and L + ZrFe2 = ZrMn2 reaction at 1853 K do not lie in one plane, which means the system does not exhibit a quasi-binary behavior.
Due to little experimental data on reactions with liquid phase, one mixing parameter L0 was added to the liquid with a temperature depended variable. Optimization was done based on the DTA data from this work, namely four melting points, temperature of the eutectic L = C14-ZrMn2 + Zr2Fe + β-Zr and temperature of L + ZrFe2 = ZrMn2 from (Ref 20). According to the obtained results, C15 + L region does not extend to the C14 rich side as it is reported in the work.[39]
5 Conclusions
The phase relations in the Fe-Mn-Zr system were investigated using XRD, SEM/EDX and DTA methods. The isothermal section at 1073 K was constructed based on experimental data and reproduced by calculations. Temperature of one invariant reaction was measured using DTA. Composition of the eutectic reaction was measured using EDX. The liquidus projection and vertical section ZrFe2-ZrMn2 were calculated.
The phase equilibria study of seven samples annealed at 1073 K for seven days resulted in establishment of the phase fields C14 + β-Zr, C14 + γ-(Fe,Mn), C15 + C14 and C14 + Zr2Fe + β-Zr. Experimental results showed that the homogeneity range of Zr2Fe has a 6 at.% Mn extension into the ternary region. Based on both experimental and modeling results, C15-C14 section was revealed to be not a quasi-binary system. The width of this section was evaluated from the measurements of the C14 phase in the samples. EBSD investigation did not confirm presence of a C36 Laves phase. Based on experimental data, a eutectic reaction Liquid ↔ C14 + Zr2Fe + β-Zr was determined to occur at 1190 K and 73/21.5/5.5 at.% Zr/Fe/Mn composition. DTA measurements of the sample #1 and #6 indicated melting points at 1901 K and 1846, respectively, and these data were used for the parameter optimization. Using all obtained experimental results and phase equilibria data from literature,[20] thermodynamic parameters for the Fe-Mn-Zr system were obtained. Resulting thermodynamic description reproduces all the experimental results very well. Current database can be used for further thermodynamic modelling of the high-order systems and hydrogen storage materials.
References
C.D. Rabadia, Y.J. Liu, S.F. Jawed et al., Improved Deformation Behavior in Ti-Zr-Fe-Mn Alloys Comprising the C14 Type Laves and β Phases, Mater. Des., 2018, 160, p 1059-1070.
S. Scudino, P. Donnadieu, K.B. Surreddi et al., Microstructure and Mechanical Properties of Laves Phase-Reinforced Fe-Zr-Cr Alloys, Intermetallics, 2009, 17, p 532-539.
G. Li, and N. Nishimiya, Phase Relations of Zr(FexMn1−x)2-H2 Systems Studied by XRD and Isothermal-Calorimetric Measurement, J. Alloys Compd., 2004, 375, p 205-211.
H. Biermann, U. Martin, C.G. Aneziris et al., Microstructure and Compression Strength of Novel TRIP-Steel/Mg-PSZ Composites, Adv. Eng. Mater., 2009, 43, p 1000-1006.
S. Martin, S. Richter, S. Decker et al., Reinforcing Mechanism of Mg-PSZ Particles in Highly-Alloyed TRIP Steel, Steel Res. Int., 2011, 82, p 1133-1140.
I. Saenko, A. Kuprava, A. Udovsky, and O. Fabrichnaya, Heat Capacity Measurement of Zr2Fe and Thermodynamic Re-assessment of the Fe-Zr System, Calphad, 2019, 66, p 101625.
B.O. Mukhamedov, I. Saenko, A.V. Ponomareva et al., Thermodynamic and Physical Properties of Zr3Fe and ZrFe2 Intermetallic Compounds, Intermetallics, 2019, 109, p 189-196.
A. Kuprava, I. Saenko, and O. Fabrichnaya, Heat Capacity Measurement of C14-ZrMn2 and Thermodynamic Re-assessment of the Mn-Zr system, Calphad, 2020, 68, p 101745.
V.T. Witusiewicz, F. Sommer, and E.J. Mittemeijer, Reevaluation of the Fe-Mn Phase Diagram. Wit 2004, J. Phase Equilib. Diffus., 2004, 25, p 346-354.
W. Huang, An Assessment of the Fe-Mn System, Calphad, 1989, 13, p 243-252.
B.-J. Lee and D.N. Lee, A Thermodynamic Study on the Mn-C and Fe-Mn Systems, Calphad, 1989, 13, p 345-354.
C.-M. Li, F. Sommer, and E.J. Mittemeijer, Characteristics of the γ→α Transformation in Fe-Mn Alloys, Mater. Sci. Eng. A, 2002, 325, p 307-319.
V.T. Witusiewicz, F. Sommer, and E.J. Mittemeijer, Enthalpy of Formation and Heat Capacity of Fe-Mn Alloys, Metall. Mater. Trans. B, 2003, 34, p 209-223.
T.B. Massalski, Ed.-in-Chief, Binary Alloy Phase Diagrams, (American Society for Metals, Metals Park, Ohio, 1986).
F. Stein, G. Sauthoff, and M. Palm, Experimental Determination of Intermetallic Phases, Phase Equilibria, and Invariant Reaction Temperatures in the Fe-Zr System, JPE, 2002, 23, p 480-494.
H.J. Wallbaum, Ergebnisse der Röntgenographischen Strukturuntersuchung von Legierungen der Zusammensetzung AB2 der Eisenmetalle mit Titan, Zirkon, Niob und Tantal (1), Z. Krist. Cryst. Mater., 1941, 103, p 391-402.
C.P. Gazzara, R.M. Middleton, R.J. Weiss, and E.O. Hall, A Refinement of the Parameters of α-Manganese, Acta Cryst., 1967, 22, p 859-862.
C.B. Shoemaker, D.P. Shoemaker, T.E. Hopkins, and S. Yindepit, Refinement of the Structure of β-Manganese and of a Related Phase in the Mn-Ni-Si System, Acta Crystallogr. B Struct. Sci., 1978, 34, p 3573-3576.
K. Kanematsu, Magnetism and Crystal Structure of Zirconium Compound with Laves Structure, J. Appl. Phys., 1968, 39, p 465-466.
V.V. Pet’kov, Interaction of Laves Phases in Zr(Fe Co, Ni)2-ZrMn2, Izv. Akad. Nauk. SSSR Met., 1972, 5, p 155-157.
K. Kanematsu, Magnetic Moment in Laves Phase Compound. II. Zr(Fe1–xMnx)2 and Zr(Fe1–xCox)2, J. Phys. Soc. Jpn., 1971, 31, p 1355-1360.
L. Lutterotti, Maud: A Rietveld Analysis Program Designed for the Internet and Experiment Integration, Acta Crystallogr. A Found. Crystallogr., 2000, 56, p s54-s54.
P.D.B. Fischer, S. Martin, A. Walnsch et al., Nanoscale Twinning in Fe-Mn-Al-Ni Martensite: A Backscatter Kikuchi Diffraction Study, J. Appl. Cryst., 2021, 54, p 54-61.
J.-O. Andersson, T. Helander, L. Höglund et al., Thermo-Calc & DICTRA, Computational Tools for Materials Science, Calphad, 2002, 26, p 273-312.
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics: The Calphad Method. Cambridge University Press, New York, 2007.
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15, p 317-425.
M. Hillert and M. Jarl, A Model for Alloying in Ferromagnetic Metals, Calphad, 1978, 2, p 227-238.
O. Redlich and A.T. Kister, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40, p 345-348.
M. Hillert, The Compound Energy Formalism, J. Alloys Compd., 2001, 320, p 161-176.
Y.B. Kuzma, V.Y. Markiv, Y.V. Voroshilov, and R.V. Skolozdra, X-Ray Structural Investigation of Some Allloys of the Zr-Fe and Zr-Co Systems, Inorg. Mater., 1966, 2, p 222-225.
K.H.J. Buschow, Thermal Stability and Magnetic Properties of Amorphous Zr1-xFex alloys, J. Less-Common Met., 1981, 79, p 243-253.
A. Winkelmann, C. Trager-Cowan, F. Sweeney et al., Many-Beam Dynamical Simulation of Electron Backscatter Diffraction Patterns, Ultramicroscopy, 2007, 107, p 414-421.
X.-Q. Chen, W. Wolf, R. Podloucky et al., Ab Initio Study of Ground-State Properties of the Laves-Phase Compound ZrMn2, Phys. Rev. B, 2005, 66, p 054440.
A. Kawsar, A. Arya, P.S. Gosh et al., A First Principles Study of Cohesive, Elastic and Electronic Properties of Binary Fe-Zr Intermetallics, Comput. Mater. Sci., 2016, 112, p 52-66.
N. Zou, H.-J. Lu, P.-W. Gu et al., Thermodynamic Assessment and Glass Forming Ability Prediction of the Zr-Fe-Cu System, Calphad, 2019, 64, p 175-184.
S. Wang, K. Wang, G. Chen et al., Thermodynamic Modeling of Ti-Fe-Cr Ternary System, Calphad, 2017, 56, p 160-168.
S. Gorsse and G.J. Shiflet, A Thermodynamic Assessment of the Cu-Mg-Ni Ternary System, Calphad, 2002, 26, p 63-83.
G. Ghosh, Thermodynamic and Kinetic Modeling of the Cr-Ti-V System, J. Phase Equilib., 2002, 23, p 310-328.
N. Leburn and P. Perrot, Fe-Mn-Zr Ternary Phase Diagram Evaluation, MSI Eureka (2008) Ternary Evaluations.
Acknowledgments
The authors thank the German Research Foundation (DFG) for funding Sub-project C2 within the Collaborative Research Center SFB 799 Trip-Matrix Composites. In addition, we thank B. Wahl and Dr. C. Schimpf (TU-Freiberg) for technical contribution.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuprava, A., Saenko, I., Fischer, P. et al. Experimental Investigation of Phase Relations and Thermodynamic Modelling of the Fe-Mn-Zr System. J. Phase Equilib. Diffus. 44, 86–101 (2023). https://doi.org/10.1007/s11669-022-01019-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-022-01019-7