Abstract
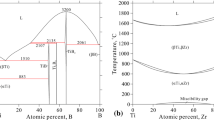
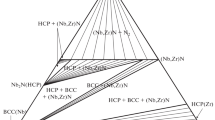
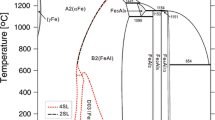
Thermodynamic assessment of the ternary system B-Hf-Zr has been conducted by modeling the Gibbs energy of all individual phases using the CALPHAD (CALculation of PHAse Diagrams) approach. There is no ternary compound in this system. The individual solution phases, i.e., liquid, (βHf,βZr), HfB and (Hf,Zr)B2 have been modeled. The modeling covers the whole composition and temperature ranges. The Gibbs energies of HfB2 and HfB in the B-Hf system were reassessed using the two-sublattice models (B,Hf)1(B,Hf)2 and (Hf)1(B,Hf)1, respectively. A set of self-consistent thermodynamic parameters for the B-Hf-Zr system was obtained by considering the phase diagram data in the ternary system. Comprehensive comparisons between the calculated and measured phase diagram and thermodynamic data show that the experimental information was satisfactorily accounted for by the present thermodynamic description. The liquidus projection and reaction scheme of the B-Hf-Zr system are also presented.










Similar content being viewed by others
References
S. Bajpai, R. Kundu, and K. Balani, Effect of B4C Reinforcement on Microstructure, Residual Stress, Toughening and Scratch Resistance of (Hf,Zr)B2 Ceramics, Mater. Sci. Eng. A, 2020, 796(7), p 140022.
F. Monteverde, F. Saraga, and M. Gaboardi, Compositional Disorder and Sintering of Entropy Stabilized (Hf,Nb,Ta,Ti,Zr)B2 Solid Solution Powders, J. Eur. Ceram. Soc., 2020, 40(12), p 3807–3814.
J. Yin, B.H. Zhang, X.J. Liu, and Z.R. Huang, Pressurelessly Densified (Zr,Hf)B2-SiC Ceramics by Co-Doping Hafnium-Boron Carbides, J. Alloys Compd., 2017, 727(15), p 706–710.
L. Silvestroni, and D. Sciti, Effects of MoSi2 Additions on the Properties of Hf– and Zr–B2 Composites Produced by Pressureless Sintering, Scripta Mater., 2007, 57, p 165–168.
M. Brochu, B. Gauntt, T. Zimmerly, A. Ayala, and R. Loehman, Fabrication of UHTCs by Conversion of Dynamically Consolidated Zr+B and Hf+B Powder Mixtures, J. Am. Ceram. Soc., 2008, 91(9), p 2815–2822.
Y.F. Pan, C. Zhang, J.X. Zhang, L. Huang, X.Y. Yang, Y. Du, and F.H. Luo, Thermodynamic Modeling of the B-Ti-Zr System over the Whole Composition and Temperature Ranges, J. Phase Equilib. Diffus., 2019, 40, p 364–374.
J.O. Andersson, T. Helander, L. Hoglund, P. Shi, and B. Sundman, Thermo-Calc & DICTRA Computational Tools for Materials Science, Calphad, 2002, 26(2), p 273–312.
L. Zhang, I. Steinbach, and Y. Du, Phase-Field Simulation of Diffusion Couples in the Ni–Al System, Int. J. Mater. Res., 2011, 102(4), p 371–380.
P. Rogl, and P.E. Potter, A Critical Review and Thermodynamic Calculation of the Binary System: Hafnium-Boron, Calphad, 1988, 12(3), p 207–218.
E. Rudy and S. Windisch, Ternary Phase Equilibriums in Transition Metal-Boron-Carbon-Silicon Systems. Technical Report No. AFML-TR-65-2, Part I, Volume IX, 1965
H. Bittermann, and P. Rogl, Critical Assessment and Thermodynamic Calculation of the Ternary System Boron-Hafnium-Titanium (B-Hf-Ti), J. Phase Equilib., 1997, 18(1), p 24–47.
V.T. Witusiewicz, A.A. Bondar, U. Hecht, O.A. Potazhevska, and T. Ya Velikanova, Thermodynamic Modelling of the Ternary B-Mo-Ti System with Refined B-Mo Description, J. Alloys Compd., 2016, 655, p 336–352.
A.S. Bolgar, and A.V. Blinder, Thermodynamic Characteristics of Hafnium and Tantalum Diboride in A Wide Temperature Range, Sov. Powder Metall. Metal Ceram., 1989, 28, p 128–131.
H.L. Schick, Thermodynamics of Certain Refractory Compounds. Academic Press, New York, 1966.
I. Barin, Thermochemical Data of Pure Substances, 3rd edn. Wiley-VCH Verlag Gmbh, Weinheim, 1995.
R. Loehman, E. Corral, H.P. Dumm, P. Kotula, and R. Tandon, Ultra High Temperature Ceramics for Hypersonic Vehicle Applications, SAND2006-2925, Albuquerque, 2006
E.F. Westrum, and G. Feick, Heat Capacities of HfB2.035 and HfC0.968 from 5 to 350 K, J. Chem. Thermodyn., 1977, 9(3), p 293–299.
R. Mezaki, E.W. Tilleux, D.W. Barnes, and J.L. Margrave, High-Temperature Thermodynamic Properties of Some Refractory Borides, Thermodyn. Nucl. Mater., IAEA Publications Proceeding Series, Vienna, 1962, pp 775–788
C.D. Pears, S. Oglesby, and D.S. Nell, The Thermal Properties of Twenty-Six Solid Materials to 5000 °C or Their Destruction Temperatures, Tech. Report ASD-TDR-62-765, Wright-Patterson A.F.B.,OH, 1963
Y. Pan, H.W. Huang, X. Wang, and Y.H. Lin, phase Stability and Mechanical Properties of Hafnium Borides: A First-Principles Study, Comput. Mater. Sci., 2015, 109, p 1–6.
C.W. Xie, Q. Zhang, H.A. Zakaryan, H. Wan, N. Liu, A.G. Kvashnin, and A.R. Oganov, Stable and Hard Hafnium Borides: A First-Principles Study, J. Appl. Phys., 2019, 125(20), p 205109.
L.A. Mcclaine, Thermodynamic and Kinetic Studies for a Refractory Material Program, Tech. Report No. ASD-TDR-62-204, Part III, Wright-Patterson A.F.B., OH, 1964
E.P. Kitpichev, Y.I. Rubtsov, T.V. Sorokina, and V.K. Prokudina, Standard Enthalpies of Formation of Some Group IV-V Element Borides, J. Phys. Chem., 1979, 53(8), p 1128–1130.
V.M. Maslov, A.S. Neganov, I.P. Borovinskaya, and A.G. Merzhanov, Self-Propagating High-Temperature Synthesis as a Method of Determining Heats of Formation of Refractory Compounds, Fiz. Goreniya Vzryva, 1978, 14(6), p 73–82.
P.J. Spencer, O. Kubaschewski-von Goldbeck, R. Ferro, R. Marazza, K. Girgis, and O. Kubaschewski, Hafnium, Physico-Chemical Properties of its Compounds and Alloys, K.L. Komarek, Ed., Atomic Energy Review, Special Issue 8, IAEA, Vienna, 1981
G.K. Johnson, E. Greenberg, J.L. Margrave, and W.N. Hubbard, Fluorine Bomb calorimetry, Enthalpies of Formation of the diborides of zirconium and hafnium, J. Chem. Eng. Data, 1967, 12(1), p 137–141.
H.L. Schick, Thermodynamics of Certain Refractory Compounds, Academic Press, New York, 1966, 1, p 240-247
F.W. Glaser, and B. Post, Trans. AIME, 1953, 197, p 1117–1118.
E. Rudy and S. Windisch, Ternary Phase Equilibriums in Transition Metal-Boron-Carbon-Silicon Systems. Technical Report No. AFML-TR-65-2, Part I, Volume VIII, 1966
K.P. Portnoi, V.M. Romashov, and L.I. Vyroshina, Constitution Diagram of the System Zirconium-Boron, Poroshkov. Metall., 1970, 91, p 68–71.
K.I. Portnoi, and V.M. Romashov, Binary Constitution Diagrams of Systems Composed of Various Elements and Boron - A Review, Poroshkov. Metall., 1972, 5, p 48–56.
H. Nowotny, E. Rudy, and F. Benesovsky, Investigations in the Systems: Zirconium-Boron-Carbon and Zirconium-Boron-Nitrogen, Monatsh Chem., 1960, 91, p 963–974.
O.I. Shulishova, and I.A. Shcherbak, Superconductivity of the Borides of Transitions and Rare-Earth Metals, Inorg. Mater., 1967, 3(8), p 1304–1306.
J.S. Haggerty, J.L. O’Brien, and J.F. Wenckus, Growth and Characterization of Single Crystal ZrB2, J. Cryst. Growth, 1968, 3(4), p 291–294.
Y. Champion, and S. Hagege, A Study of Composite Interfaces in the Zr-ZrB2 System, J. Mater. Sci. Lett., 1992, 11, p 290–293.
Y. Champion, and S. Hagege, Experimental Determination and Symmetry Related Analysis of Orientation Relationships in Heterophase Interfaces: a Case Study in the Zr-B System, Acta Mater., 1996, 44(10), p 4169–4179.
Y. Champion, and S. Hagege, Structural Analysis of Phases and Heterophase Interfaces in the Zirconium-Boron System, J. Mater. Sci., 1998, 33, p 4035–4041.
H. Bittermann, and P. Rogl, Critical Assessment and Thermodynamic Calculation of the Ternary System C-Hf-Zr (Carbon-Zirconium-Hafnium), J. Phase Equilib., 2002, 23(3), p 218–235.
D.P. Harmon, Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems Technical Report No. AFML-TR-65-2, Part II, Volume XI, 1965
E. Rudy, Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems. Technical Report No. AFML-TR-65-2, Part V, 1969
G. Cacciamani, P. Riani, and F. Valenza, Equilibrium Between MB2 (M = Ti, Zr, Hf) UHTC and Ni: A Thermodynamic Database for the B-Hf-Ni-Ti-Zr System, Calphad, 2011, 35, p 601–619.
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15(4), p 317–425.
O. Redlich, and A.T. Kister, Thermodynamics of Nonelectrolytic Solutions. Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40, p 84–88.
Y.M. Muggianu, M. Gambino, and J.P. Bros, Enthalpies of Formation of Liquid Alloys Bismuth-Gallium-Tin at 723.Deg.K Choice of an Analytical Representation of Integral and Partial Excess Functions of Mixing, J. Chim. Phys. Phys. Chim. Biol., 1975, 72(1), p 83–88.
M. Hillert, and L.-I. Staffansson, The Relationship Between Gibbs Free Energy and the Intersection of the Liquidi in Phase Diagrams of Reciprocal Systems, Metall. Trans. B, 1975, 6B, p 613–616.
P. Rogl, J. Vřešťál, T. Tanaka, and S. Takenouchi, The B-rich Side of the B-C Phase Diagram, Calphad, 2014, 44, p 3–9.
B. Sundman, B. Jansson, and J.O. Andersson, The Thermo-Calc Databank System, Calphad, 1985, 9, p 153–190.
Y. Du, R. Schmid-Fetzer, and H. Ohtani, Thermodynamic Assessment of the V-N System, Z. Metallkd., 1997, 88, p 545–556.
Acknowledgments
The financial support from the National Natural Science Foundation of China (51901063), the Fundamental Research Funds for the Central Universities of China (JZ2021HGTB0094 and PA2021GDGP0059) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, Y., Huang, L., Zhang, J. et al. Thermodynamic Assessment of the Ternary B-Hf-Zr System with Refined B-Hf Description. J. Phase Equilib. Diffus. 42, 864–878 (2021). https://doi.org/10.1007/s11669-021-00928-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-021-00928-3