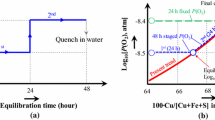
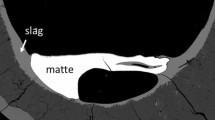
Abstract
The individual effects of Al2O3, CaO and MgO on gas/slag/matte/spinel equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) system at 1473 K (1200 °C) and p(SO2) = 0.25 atm. have been experimentally measured for a range of oxygen partial pressures and matte compositions. The experimental methodology has included the high temperature equilibration of individual samples on a spinel primary phase substrate under controlled gas atmospheres (CO/CO2/SO2/Ar), followed by rapid quenching of the equilibrium condensed phases and direct measurement of the phase compositions using electron probe x-ray microanalysis. The experimental results show that the presence of Al2O3, CaO and MgO reduce the iron, sulphur and copper concentrations in the slag phase. Present study is undertaken as part of an integrated approach involving thermodynamic modelling and experimental measurements. The experimental data are compared with predictions obtained using the current thermodynamic database for the Cu-Fe-O-S-Si-(Al, Ca, Mg) system in order to further improve thermodynamic parameters.









Similar content being viewed by others
References
W.G.I. Davenport et al., Extractive Metallurgy of Copper, 4th ed., Pergamon Press, Oxford, 2002, p 155-171
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. III. Partial Liquidus Diagram for Cu2S-FeS-FeO System, Technol. Rep. Tohoku Univ., 1955, 19(2), p 239-250
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. IV. Solubility of FeO in Copper Matte from SiO2-saturated FeO-SiO2 Slag, Technol. Rep. Tohoku Univ., 1955, 19(2), p 251-261
Kameda, M. and A. Yazawa. The Oxygen Content of Copper Mattes, in Physical Chemistry of Process Metallurgy, Part 2 (TMS Conference Proceedings, Interscience, NY, 1961)
N. Korakas, Etude thermodynamic de l’équilibre entre scories ferro-siliceuses et mattes de cuivre. Application aux problèmes posés par la formation de magnetite lors du traitement des minerais sulfurés de cuivre (Univirsité de Liège, 1964)
U. Kuxmann and F.Y. Bor, Studies on the Solubility of Oxygen in Copper Mattes under Ferric Oxide Slags Saturated with Silica, Erzmetall, 1965, 18, p 441-450
F.Y. Bor and P. Tarassoff, Solubility of Oxygen in Copper Mattes, Can. Metall. Q., 1971, 10(4), p 267-271
A. Geveci and T. Rosenqvist, Equilibrium Relations Between Liquid Copper, Iron-Copper Matte, and Iron Silicate Slag at 1250 °C, Trans. Inst. Min. Metall., 1973, 82, p C193-C201
M. Nagamori, Metal Loss to Slag: Part I. Sulfidic and Oxidic Dissolution of Copper in Fayalite Slag from Low Grade Matte, Metall. Trans. B, 1974, 5(3), p 531-538
F.J. Tavera and W.G. Davenport, Equilibrations of Copper Matte and Fayalite Slag under Controlled Partial Pressures of Sulfur Dioxide, Metall. Trans. B, 1979, 10B(2), p 237-241
G.H. Kaiura, K. Watanabe, and A. Yazawa, The Behavior of Lead in Silica-Saturated Copper Smelting Systems, Can. Metall. Q., 1980, 19(2), p 191-200
H. Jalkanen, Copper and Sulfur Solubilities in Silica-Saturated Iron Silicate Slags from Copper Mattes, Scand. J. Metall., 1981, 10(4), p 177-184
A. Yazawa, S. Nakazawa, and Y. Takeda, Distribution Behavior of Various Elements in Copper Smelting Systems, Adv. Sulfide Smelt., 1983, 1, p 99-117
R. Shimpo et al., A Study on the Equilibrium Between Copper Matte and Slag, Can. Metall. Q., 1986, 25(2), p 113-121
F.J. Tavera and E. Bedolla, Distribution of Copper, Sulfur, Oxygen and Minor Elements Between Silica-Saturated Slag, Matte and Copper-Experimental Measurements, Int. J. Miner. Process., 1990, 29(3–4), p 289-309
H. Li and W.J. Rankin, Thermodynamics and Phase Relations of the Fe-O-S-SiO2(sat) System at 1200 °C and the Effect of Copper, Metall. Trans. B, 1994, 25B(1), p 79-89
Y. Takeda, Oxygen Potential Measurement of Iron Silicate Slag–Copper–Matte System, in Proceedings International Conference on Molten Slags, Fluxes Salts ‘97, 5th (Iron and Steel Society, Warrendale, 1997)
Y. Takeda, Copper Solubility in Matte Smelting Slag, in Proceeding International Conference on Molten Slags, Fluxes Salts ‘97, 5th (Iron and Steel Society, Warrendale, 1997)
J.M. Font et al., Solubility of Copper or Nickel in Iron-Silicate Base Slag Equilibrated with Cu2s-FeS or Ni3S2-FeS Matte Under High Partial Pressures of SO2, Metall. Rev. MMIJ, 1998, 15(1), p 75-86
D. Shishin, S.A. Decterov, and E. Jak, Thermodynamic Assessment of Slag–Matte–Metal Equilibria in the Cu-Fe-O-S-Si System, J. Phase Equilib. Diffus., 2018, 39(5), p 456-475
D. Shishin, P.C. Hayes, and E. Jak. Multicomponent Thermodynamic Databases for Complex Non-ferrous Pyrometallurgical Processes, in Extraction 2018 (Springer, Ottawa, 2018).
E. Jak et al., Integrated Experimental Phase Equilibria and Thermodynamic Modelling Studies for Copper Pyrometallurgy, in 9th International Copper Conference, Kobe, Japan (2016), pp. 1316–1331
T. Hidayat et al., Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at T = 1250 °C and p(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, 49(4), p 1732-1739
Hidayat, T., et al., Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at T = 1200 °C and p(SO2) = 0.25 atm. Metall. Mater. Trans. B, 2018. 49(4): p. 1750-1765.
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and p(SO2) = 0.1 atm, Int. J. Mater. Res., 2018, 49, p 1732-1739
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and p(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2017, 48(6), p 3017-3026
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Development of Technique, Metall. Mater. Trans. B, 2017, 48(6), p 3002-3016
T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Matte/Spinel Equilibria in the Cu-Fe-O-S System at 1473 K (1200 °C) and p(SO2) = 0.25 atm, J. Phase Equilib. Diffus., 2018, 39(2), p 138-151
D. Shishin et al., Integrated Experimental and Thermodynamic Modelling Study of the Effects of Al2O3, CaO and MgO on Slag-Matte Equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) System, J. Phase Equilib. Diffus., 2018, 40, p 445-461
S. Sineva et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si-Al-Ca-Mg System in Controlled Gas Atmosphere: Experimental Results at 1473 K (1200 °C), 1573 K (1300 C) and p(SO2) = 025 atm, J. Phase Equilib. Diffus., 2020, 41, p 243-256. https://doi.org/10.1007/s11669-020-00810-8
C.W. Bale et al., FactSage Thermochemical Software and Databases, Calphad, 2002, 26(2), p 189-228
C.W. Bale et al., FactSage Thermochemical Software and Databases, 2010–2016, Calphad, 2016, 54, p 35-53
D. Shishin, P.C. Hayes, and E. Jak, Development and Applications of Thermodynamic Database in Copper Smelting, in Copper’19 Conference (Vancouver, 2019)
Acknowledgments
The authors would like to thank Australian Research Council Linkage program LP140100480, Altonorte Glencore, AngloAmerican Platinum, Atlantic Copper, Aurubis, Boliden, Olympic Dam Operation BHP Billiton, Kazzinc Glencore, Kennecott Rio Tinto, Outotec Oy (Espoo), PASAR Glencore, Umicore, and for the financial support for this study. The authors would like to thank Dr Denis Shishin and Dr Maksym Maksym Shevchenko for assistance in preparation of this paper. The authors would also like to thank the staff of the Centre for Microscopy and Microanalysis, University of Queensland for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sineva, S., Fallah-Mehrjardi, A., Hidayat, T. et al. Experimental Study of the Individual Effects of Al2O3, CaO and MgO on Gas/Slag/Matte/Spinel Equilibria in Cu-Fe-O-S-Si-Al-Ca-Mg System at 1473 K (1200 °C) and p(SO2) = 0.25 atm. J. Phase Equilib. Diffus. 41, 859–869 (2020). https://doi.org/10.1007/s11669-020-00847-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00847-9