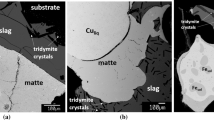
Abstract
Equilibria among the slag, matte and metal phases in the Cu-Fe-O-S-Si system are critically assessed using thermodynamic modeling. The relationships among matte grade, temperature, partial pressure of SO2, Fe/SiO2 in the slag, and the copper concentration in the slag are described by the model, as well as the concentrations of other elements in all phases. A thermodynamic database is created, which can be used for understanding and improving the pyrometallurgical production of copper. An extensive experimental dataset includes the most recent results obtained by the equilibration/quenching/EPMA analysis technique. These data allow to distinguish the physical entrainment of the matte and solid phases in the slag from chemical solubility. As a result, it is possible to describe the copper solubility in the slag with high accuracy and establish the relationship between copper and sulfur in the slag. The thermodynamic database of the present study is consistent with previously reported thermodynamic evaluations of binary, ternary and quaternary subsystems. The slag phase is modeled using the two-sublattice modified quasichemical model in the quadruplet approximation. The matte and metal liquid phases are modeled as one solution using the single-sublattice modified quasichemical model in the pair approximation.











Similar content being viewed by others
References
S.A. Degterov and A.D. Pelton, A Thermodynamic Database for Copper Smelting and Converting, Metall. Mater. Trans. B, 1999, 30B, p 661-669
E. Jak, P. Hayes, C.W. Bale, and S.A. Decterov, Application of FactSage Thermodynamic Modeling of Recycled Slags (Al2O3-CaO-FeO-Fe2O3-SiO2-PbO-ZnO) in the Treatment of Wastes from End-of-Life-Vehicles, Int. J. Mater. Res., 2007, 98, p 872-878
E. Jak, S.A. Decterov, B. Zhao, A.D. Pelton, and P.C. Hayes, Coupled Experimental and Thermodynamic Modelling Studies for Metallurgical Smelting and Coal Combustion Slag Systems, Metall. Mater. Trans. B, 2000, 31B, p 621-630
E. Jak, S.A. Decterov, P.C. Hayes, and A.D. Pelton, in Thermodynamic Modelling of the System PbO-ZnO-FeO-Fe 2 O 3 -CaO-SiO 2 for Zinc/Lead Smelting. Proceedings of 5th International Conference on Molten Slags, Fluxes and Salts, Iron and Steel Society, AIME, Sydney, 1997, pp. 621–628
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melancon, A.D. Pelton, C. Robelin, and S. Petersen, FactSage Thermochemical Software and Databases—Recent Developments, CALPHAD, 2009, 33, p 295-311
J. Chen, P.C. Hayes, and E. Jak, in Experimental Investigation of Slag/Matte/Metal/Tridymite Equilibrium in the Cu-Fe-O-S-Si System at T = 1200 °C: Development of Technique and Results. Internal Report University of Queensland, 2017
J. Chen, P.C. Hayes, and E. Jak, in Experimental Investigation of Slag/Matte/Metal/Tridymite Equilibrium in the Cu-Fe-O-S-Si System at T = 1250 and 1300 °C. Internal Report University of Queensland, 2017
T. Hidayat, A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at T = 1250 °C and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, submitted
T. Hidayat, A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at 1473 K (1200 °C) and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, 49, p 1750-1765
T. Hidayat, A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Matte/Spinel Equilibria in the Cu-Fe-O-S System at T = 1200 °C and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, submitted
T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Matte/Spinel Equilibria in the Cu-Fe-O-S System at T = 1250 °C and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, submitted
A. Fallah-Mehrjardi, T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmosphere: Experimental Results at T = 1523 K (1250 °C) and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, 49, p 1732-1739
A. Fallah-Mehrjardi, T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si System in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2017, 48, p 3017-3026
A. Fallah-Mehrjardi, T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Development of Technique, Metall. Mater. Trans. B, 2017, 48, p 3002-3016
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and P(SO2) = 0.1, 0.25, 0.6 atm, Metall. Mater. Trans. B, 2018, submitted
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si-Mg System in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and P(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, submitted
E. Jak, T. Hidayat, D. Shishin, A.F. Mehrjardi, J. Chen, and P. Hayes, in Integrated Experimental Phase Equilibria and Thermodynamic Modelling Studies for Copper Pyrometallurgy. 9th International Copper Conference, Kobe, Japan, 2016
T. Hidayat, D. Shishin, E. Jak, and S. Decterov, Thermodynamic Reevaluation of the Fe-O System, CALPHAD, 2015, 48, p 131-144
P. Waldner and A.D. Pelton, Thermodynamic Modeling of the Fe-S System, J. Phase Equilib. Diffus., 2005, 26, p 23-28
P. Waldner and A.D. Pelton, in Thermodynamic Modeling of the Cu-Fe-S System. Internal Report, Ecole Polytechnique de Montreal (Montreal, QC, Canada), 2006
D. Shishin, in Development of a Thermodynamic Database for Copper Smelting and Converting, Ph. D. Thesis, École Polytechnique de Montréal, 2013
D. Shishin and S.A. Decterov, Critical Assessment and Thermodynamic Modeling of Cu-O and Cu-O-S Systems, CALPHAD, 2012, 38, p 59-70
D. Shishin, T. Hidayat, E. Jak, and S. Decterov, Critical Assessment and Thermodynamic Modeling of Cu-Fe-O System, CALPHAD, 2013, 41, p 160-179
D. Shishin, E. Jak, and S.A. Decterov, Critical Assessment and Thermodynamic Modeling of the Fe-O-S System, J. Phase Equilib. Diffus., 2015, 36, p 224-240
D. Shishin, E. Jak, and S.A. Decterov, Thermodynamic Assessment and Database for the Cu-Fe-O-S System, CALPHAD, 2015, 50, p 144-160
T. Hidayat and E. Jak, Thermodynamic Modeling of the “Cu2O”-SiO2, “Cu2O”-CaO, and “Cu2O”-CaO-SiO2 Systems in Equilibrium with Metallic Copper, Int. J. Mater. Res., 2014, 105, p 249-257
T. Hidayat, D. Shishin, S.A. Decterov, and E. Jak, Experimental Study and Thermodynamic Re-evaluation of the FeO-Fe2O3-SiO2 System, J. Phase Equilib. Diffus., 2017, 38, p 477-492
T. Hidayat, D. Shishin, S. Decterov, and E. Jak, Critical Assessment and Thermodynamic Modeling of the Cu-Fe-O-Si System, CALPHAD, 2017, 58, p 101-114
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melancon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M.A. Van Ende, FactSage Thermochemical Software and Databases, 2010-2016, CALPHAD, 2016, 54, p 35-53
G. Lambotte, in Approche Thermodynamique de la Corrosion des Réfractaires Aluminosiliceux par le Bain Cryolithique : Modélisation Thermodynamique du Système Quaternaire Réciproque AlF 3-NaF-SiF 4-Al 2 O 3-Na 2 O-SiO 2. Ph. D. Thesis, École Polytechnique de Montréal, 2012
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. IV. Solubility of FeO in Copper Matte from SiO2-saturated FeO-SiO2 Slag, Technol. Rep. Tohoku Univ., 1955, 19, p 251-261
M. Kameda and A. Yazawa, in The Oxygen Content of Copper Mattes, ed. by G.R.S. Pierre. Proceedings of Physical Chemistry of Process Metallurgy, Part 2, TMS Conference. Proceedings of Interscience, NY, 1961, pp. 963–988
N. Korakas, Etude Thermodynamic de l’équilibre Entre Scories Ferro-siliceuses et Mattes de Cuivre. Application aux Problèmes Posés par la Formation de Magnetite Lors du Traitement des Minerais Sulfurés de Cuivre, Ph. D. Thesis, Univirsité de Liège, 1964
U. Kuxmann and F.Y. Bor, Studies on the Solubility of Oxygen in Copper Mattes under Ferric Oxide Slags Saturated with Silica, Erzmetall, 1965, 18, p 441-450
F.Y. Bor and P. Tarassoff, Solubility of Oxygen in Copper Mattes, Can. Metall. Q., 1971, 10, p 267-271
A. Geveci and T. Rosenqvist, Equilibrium Relations between Liquid Copper, Iron–Copper Matte, and Iron Silicate Slag at 1250°, Trans. Inst. Min. Metall., 1973, 82, p C193-C201
M. Nagamori, Metal Loss to Slag: Part I. Sulfidic and Oxidic Dissolution of Copper in Fayalite Slag from Low Grade Matte, Metall. Trans. B, 1974, 5B, p 531-538
F.J. Tavera and W.G. Davenport, Equilibrations of Copper Matte and Fayalite Slag under Controlled Partial Pressures of Sulfur Dioxide, Metall. Trans. B, 1979, 10B, p 237-241
G.H. Kaiura, K. Watanabe, and A. Yazawa, The Behavior of Lead in Silica-Saturated Copper Smelting Systems, Can. Metall. Q., 1980, 19, p 191-200
Y. Takeda, in Copper Solubility in Matte Smelting Slag. Proceedings of International Conference on Molten Slags, Fluxes Salts ‘97, 5th, Iron and Steel Society Warrendale, PA, 1997, pp. 329–339
W.H. MacLean, Liquid Phase Relations in the FeS-FeO-Fe3O4-SiO2 System, and Their Application in Geology, Econ. Geol., 1969, 64, p 865-884
D. Dilner and M. Selleby, Thermodynamic Description of the Fe-Ca-O-S System, CALPHAD, 2017, 57, p 118-125
Y. Jo, H.-G. Lee, and Y.-B. Kang, Thermodynamics of the MnO-FeO-MnS-FeS-SiO2 System at SiO2 Saturation Under Reducing Condition: Immiscibility in the Liquid Phase, ISIJ Int., 2013, 53, p 751-760
A.D. Pelton, P. Chartrand, and G. Eriksson, The Modified Quasichemical Model IV—Two Sublattice Quadruplet Approximation, Metall. Mater. Trans. A, 2001, 32, p 1409-1415
R. Piao, H.-G. Lee, and Y.-B. Kang, Experimental Investigation of Phase Equilibria and Thermodynamic Modeling of the CaO-Al2O3-CaS and the CaO-SiO2-CaS Oxysulfide Systems, Acta Mater., 2013, 61, p 683-696
H. Jalkanen, Copper and Sulfur Solubilities in Silica-Saturated Iron Silicate Slags from Copper Mattes, Scand. J. Metall., 1981, 10, p 177-184
A. Yazawa, S. Nakazawa, Y. in Takeda, Distribution Behavior of Various Elements in Copper Smelting Systems, ed by H.Y. Sohn, D.B. George, A.D. Zunkel. Proceedings of International Sulfide Smelting Symposium on Extr. Process Metall. Meet. Metall. Soc, Advance Sulfide Smelting, AIME, 1983, pp. 99–117
R. Shimpo, S. Goto, O. Ogawa, and I. Asakura, A Study on the Equilibrium Between Copper Matte and Slag, Can. Metall. Q., 1986, 25, p 113-121
F.J. Tavera and E. Bedolla, Distribution of Copper, Sulfur, Oxygen and Minor Elements Between Silica-Saturated Slag, Matte and Copper—Experimental Measurements, Int. J. Miner. Process., 1990, 29, p 289-309
H. Li and W.J. Rankin, Thermodynamics and Phase Relations of the Fe-O-S-SiO2(sat) System at 1200 °C and the Effect of Copper, Metall. Trans. B, 1994, 25B, p 79-89
Y. Takeda, in Oxygen Potential Measurement of Iron Silicate Slag–Copper–Matte System. Proceedings of International Conference on Molten Slags, Fluxes Salts ‘97, 5th, Iron and Steel Society Warrendale, PA, 1997, pp. 735–743
J.M. Font, G. Roghani, M. Hino, and K. Itagaki, Solubility of Copper or Nickel in Iron-Silicate Base Slag Equilibrated with Cu2S-FeS or Ni3S2-FeS Matte Under High Partial Pressures of SO2, Metall. Rev. MMIJ, 1998, 15, p 75-86
A. Yazawa, Thermodynamic Considerations of Copper Smelting, Can. Metall. Q., 1974, 13, p 443-453
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. I. Partial Liquidus Diagram for FeS-FeO-SiO2 System, Technol. Rep. Tohoku Univ., 1953, 18, p 40-58
Y.-B. Kang and A. Pelton, Thermodynamic Model and Database for Sulfides Dissolved in Molten Oxide Slags, Metall. Mater. Trans. B, 2009, 40, p 979-994
M.M. Nzotta, D. Sichen, and S. Seetharaman, Sulfide Capacities of FeO-SiO2, CaO-FeO, and FeO-MnO Slags, ISIJ Int., 1999, 39, p 657-663
S.R. Simeonov, R. Sridhar, and J.M. Toguri, Sulfide Capacities of Fayalite-Base Slags, Metall. Trans. B, 1995, 26B, p 325-334
M.G. Park, Y. Takeda, and A. Yazawa, Equilibrium Relations Between Liquid Copper, Matte and Calcium Ferrite Slag at 1250 °C, Tohoku Daigaku Senko Seiren Kenkyusho Iho, 1983, 39, p 115-122
C. Acuna and A. Yazawa, Mutual Dissolution Between Matte and Ferrite Slags, Trans. Jpn. Inst. Met., 1986, 27, p 881-889
G. Roghani, M. Hino, and K. Itagaki, Phase Equilibrium Between Calcium Ferrite Slag and Copper Matte at 1523 K Under High Partial Pressures of SO2, Mater. Trans. JIM, 1996, 37, p 1431-1437
F. Sehnalek and I. Imris, in Slags from Continuous Copper Production, ed. by M.J. Jones. Proceedings of International Symposium on Institution of Mining and Metallurgy, Advances in Extractive Metallurgy and Refining, London, England, 1972, pp. 39–62
P. Spira and N. Themelis, Solubility of Copper in Slags, J. Met., 1969, 21, p 35-42
E.-B. Johansen, T. Rosenqvist, and P.T. Torgersen, Thermodynamics of Continuous Copper Smelting, J. Met., 1970, 22, p 39-47
J.M. Font, Y. Takeda, and K. Itagaki, Phase Equilibrium Between Iron–Silicate Base Slag and Nickel–Iron Matte at 1573 K Under High Partial Pressures of SO2, Mater. Trans., 1998, 39, p 652-657
P. Tan, in CuModel—A Thermodynamic Model and Computer Program of Copper Smelting and Converting Processes and Its Industrial Applications, ed. by M.E. Schlesinger. EPD Congress 2004, Proceedings of Sessions and Symposia held during the TMS Annual Meeting, Charlotte, NC, United States, Mar 14–18, 2004, Minerals, Metals and Materials Society, Warrendale, PA, 2004, pp. 411–422
A.D. Pelton, Thermodynamic Models and Databases for Slags, Fluxes and Salts, Trans. Inst. Min. Metall. Sect. C, 2005, 114, p 172-180
D. Shishin, T. Hidayat, S. Decterov, and E. Jak, in Thermodynamic Modelling of Liquid Slag–Matte–Metal Equilibria Applied to the Simulation of the Peirce-Smith Converter. Proceedings of 10th International Conference on Molten Slags, Fluxes and Salts, Seattle, Seattle, USA, 2016
D. Shishin, T. Hidayat, E. Jak, S. Decterov, and G.V. Belov, Thermodynamic Database for Pyrometallurgical Copper Extraction, in Proceedings of Copper’2016, Kobe, Japan, 2016, p. 12
C.J.B. Fincham and F.D. Richardson, The Behaviour of Sulphur in Silicate and Aluminate Melts, Proc. R. Soc. (Lond.), 1954, 223, p 40-62
G. Eriksson and A.D. Pelton, Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 Systems, Metall. Trans., 1993, 24, p 807-816
Acknowledgments
The authors would like to thank Australian Research Council, linkage project LP140100480 “Creating sustainable copper supplies by using innovative high temperature chemical processing of highly complex impure ores and recycled materials”. We appreciate the financial and technical support by the consortium of copper producers: Umicore NV, Aurubis AG, Kazzinc Ltd (Glencore), Outotec Oy, Complejo Metalúrgico Altonorte, Atlantic Copper, BHP Billiton Olympic Dam Corporation, PASAR (Glencore), Anglo American Platinum, Kennecott (Rio Tinto).
Author information
Authors and Affiliations
Corresponding author
Additional information
This invited article is part of a special issue of the Journal of Phase Equilibria and Diffusion in honor of Prof. Zhanpeng Jin’s 80th birthday. The special issue was organized by Prof. Ji-Cheng (JC) Zhao, The Ohio State University; Dr. Qing Chen, Thermo-Calc Software AB; and Prof. Yong Du, Central South University.
Rights and permissions
About this article
Cite this article
Shishin, D., Jak, E. & Decterov, S.A. Thermodynamic Assessment of Slag–Matte–Metal Equilibria in the Cu-Fe-O-S-Si System. J. Phase Equilib. Diffus. 39, 456–475 (2018). https://doi.org/10.1007/s11669-018-0661-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0661-0