Abstract
Two different methods were employed to prepare samples of the ternary intermetallic phase: AlNi2Ti, and of Al0.4Fe0.5Ni0.1, Al0.4Fe0.3Ni0.3, Al0.4Fe0.1Ni0.5 solid solutions, respectively. Alloys were then subjected to heat treatment for homogenization. Phase identification was made by XRD phase analysis and by the SEM BSE technique, and chemical composition was determined using the EDS method. Heat capacity measurements were made within a temperature range from 100 to 600 K using the DSC technique. Results were listed in tables and then fitted to a Kelley equation; an example of C p vs. T curve is given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various combinations of alloying elements from Al-Fe-Ni-Ti system are used among others in “heavy duty” construction material applications. The properties of the material depend on several factors like chemical composition, phase constitution, stability, and transitions. On the other hand, various material properties may be considerably improved by proper heat treatment, but to do that, information is needed about phase equilibria of the material of interest in a certain temperature range. The information mentioned above are available from a reliable phase diagram. Phase diagrams are currently constructed by the CALPHAD method,[1,2] and are based on the various kinds of thermodynamic and constitutional data of a material under consideration. Accuracy of the phase diagram calculated by CALPHAD approach depends in turn on the quality and amount of data used, therefore all the experimental works on thermodynamics and constitution of various alloy systems are of great interest for materials scientists and engineers.
Heat capacities of solid ternary alloys are relatively easy to measure, provided single-phase, homogeneous material is available, therefore special attention was paid to the proper sample preparation. Composition of alloys were chosen on the basis of phase diagrams presented in ternary alloys [3] for Al-Fe-Ni and Ding et al.[4] for Al-Ni-Ti systems.
Sample Preparation
Alloys were prepared from metals of at least 99.5% purity supplied by Alfa Aesar.
Samples corresponding to formulae: Al0.4Fe0.5Ni0.1, Al0.4Fe0.3Ni0.3, Al0.4Fe0.1Ni0.5 were obtained by induction melting of the appropriate amounts of metals in alumina crucibles at about 1500 °C for 30-50 s, and then cooled quickly. It was found from EDS analysis, that Al0.4Fe0.5Ni0.1 is homogeneous, while other alloys were subjected to homogenization in the evacuated quartz capsules at 1000 °C for 24 h. Examples of microstructure of an “as cast” Al0.4Fe0.3Ni0.3 alloy sample (a) and after homogenization (b) are shown in Fig. 1.
Alloy of composition AlNi2Ti was prepared by melting components in a magnesia crucible in a resistance furnace under argon of high purity (glove box): oxygen <0.1 ppm, moisture <1 ppm, nitrogen <2 ppm. Homogenization was carried out directly after melting: temperature was lowered 100° below phase melting point, to 1,100 °C, and kept for 8 h, then temperature was lowered again during 12 h until 750 °C was reached, and sample was heated back to temperature ~1100 °C. This procedure was repeated 15 times, and then samples were subjected once again to EDS analysis, to confirm homogeneity.
Measurement Procedures
Measurements were carried out within the relatively low-temperature range from 100 to 600 K using a differential scanning calorimeter, TA Instruments Q1000; T TMzero technology and calibration for a sapphire single crystal were employed. T TMzero technology introduces a new sensor for Q series 100 and 1000 of TA Instruments enabling an improvement in the reproducibility of the baseline of the DSC instrument.
Samples of about 50 mg were placed in aluminum crucibles and protected by an atmosphere of pure argon or helium. Heating rate of 20°/min, although high, was found optimal after preliminary runs; for lower rates the reproducibility of results was worse. Specific heat capacity values were directly derived by the use of standard software of the instrument.
Results
A Kelley equation is usually used to describe temperature dependence of specific heat capacity on temperature in its original form:
Sometimes, for a better fit to experimental data, additional terms are used like T −0.5, T −1, or T −3. Analysis of our experimental data resulted in the introduction of additional terms, as below:
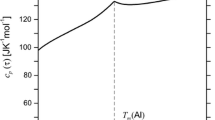
Measurement results are listed in Tables 1 and 2, and fitted to a Kelley equation in the above form; equation coefficients are given in Table 3. An example of C p versus T curve for the AlNi2Ti intermetallic phase is shown in Fig. 2.
Conclusions
Unique methods of preparation and heat treatment were employed to obtain single-phase, homogeneous alloy samples.
Specific heat capacities of ternary Al-Fe-Ni and Al-Ni-Ti alloys were measured for the first time ever.
Negative deviation from Neumann-Kopp rule was observed in all cases investigated.
References
N. Saunders and A.P. Miodownik, CALPHAD (A Comprehensive Guide), Elsevier, London, 1998
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics, Cambridge University Press, Cambridge, UK, 2007
G. Petzow and G. Effenberg, Ternary Alloys, VCH Publishers, New York, 1992
J.J. Ding, P. Rogl, and H. Schmidt, Phase Relations in the Al-Rich Corner of the Ti-Ni-Al System, J. Alloys Compd., 2001, 379, p 317-318
Acknowledgments
This study was carried out in the frame of the European Project “COST Action 535: Thermodynamics of Alloyed Aluminides-THALU.” Financial support from Polish Ministry of Science and Informatics under grant Nr 62/E-88/SPB/COST/T-08/DWM 122/2004-2007 is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Onderka, B., Sypien, A., Wierzbicka-Miernik, A. et al. Specific Heat Capacities of Some Ternary Aluminides. J. Phase Equilib. Diffus. 32, 39–41 (2011). https://doi.org/10.1007/s11669-010-9822-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-010-9822-5