Abstract
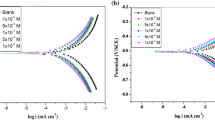
In the present study, corrosion behavior of mild steel in the presence of 4-chloro,8-(trifluoromethyl)quinoline (CTQ) was experimentally investigated. Investigator conducted the experiments using electrochemical methods such as polarization and electrochemical impedance techniques at 303–333 K. The obtained results suggested that CTQ is a good corrosion inhibitor for mild steel in 1 M hydrochloric acid medium. The CTQ adsorbed over metal surface obeys Langmuir adsorption isotherm. The thermodynamic parameters such as −∆G0ads , −∆H0ads , −∆S0ads were measured. The relationship between temperature and corrosion rate was explained by activation parameters.









Similar content being viewed by others
References
B.M. Praveen, T.V. Venkatesha, Metol as a corrosion inhibitor for steel. Int. J. Electrochem. Sci. 4, 267–275 (2009)
N. Shankaresha, T.V. Venkatesha, G. Achary, B.M. Praveen, Y.A. Naik, Corrosion behaviour of surface modified steel by condensation product. Bull. Electrochem. 23, 123–127 (2007)
B.S. Shylesha, T.V. Venkatesha, G. Harshini, B.M. Praveen, Veratraldehyde as corrosion inhibitor for mild steel in different acid medium. J. Chem. Chem. Eng. 4, 1934–7375 (2010)
B.S. Shylesha, T.V. Venkatesha, B.M. Praveen, Corrosion Inhibition studies of mild steel by new inhibitor in different corrosive medium. Res. J. Chem. Sci 1, 46–50 (2011)
R.A. Prabhu, T.V. Venkatesha, A.V. Shanbhag, B.M. Praveen, G.M. Kulkarni, R.A. Kalkhambkar, Quinol-2-thione compounds as corrosion inhibitors for mild steel in acid solution. Mater. Chem. Phys. 108, 283–289 (2008)
U.S. Umoren, R.A. Ekane, Inhibition of mild steel corrosion in H2SO4 using exudate gum from pachylobusedulis and synergistic potassium halides additives. Chem. Eng. Commun. 197, 1339–1356 (2010)
A.K. Singh, M.A. Quraishi, Effect of cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 52, 152–160 (2010)
P.L. Dusit, U. Pakawadee, P. Sutthivaiyakit, Tryptamine as a corrosion inhibitor of mild steel in hydrochloric acid solution. Corros. Sci. 52, 30–36 (2010)
I.J. Bhat, V.D.P. Alva, Inhibition effect of nevirapine an antiretroviral on the corrosion of mild steel under acidic condition. J. Kor. Chem. Soc. 55, 835–841 (2011)
N. Hebbar, B.M. Praveen, B.M. Prasanna, T.V. Venkatesha, S.B. Abd Hamid, Adsorption, thermodynamic, and electrochemical studies ofketosulfide for mild steel in acidic medium. J. Adhes. Sci. Technol. 29, 2692–2708 (2015)
B.M. Prasanna, B.M. Praveen, N. Hebbar, T.V. Venkatesha, H.C. Tandon, Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. I&EC Res. 53, 8436–8444 (2014)
B.M. Prasanna, B.M. Praveen, N. Hebbar, Theoretical and experimental approach of inhibition effect by sulfamethoxazole on mild steel corrosion in 1 M HCl. Surf. Int. Anal. (2018). https://doi.org/10.1002/sia.6457
N. Hebbar, B.M. Praveen, B.M. Prasanna, H.P. Sachin, Anticorrosion potential of flectofenine on mild steel in hydrochloric acid media: experimental and theoretical study. J. Fail. Anal. Prev. 18, 371–381 (2018)
B.M. Prasanna, B.M. Praveen, N. Hebbar, T.V. Venkatesha, H.C. Tandon, S.B. Abd Hamid, Electrochemical study on inhibitory effect of Aspirin on mild steel in 1 M hydrochloric acid. J. Ass. Arab. Univ. Appl. Sci. 22, 62–69 (2017)
D.G. Lucero, O. Xomet, R.M. Palou, V. Natalya Likhanova, D.-A. Marco, A.V. Garibay-Febles, Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 50, 7129–7140 (2011)
A. Khadraoui, A. Khelifa, H. Hamitouche, R. Mehdaoui, Inhibitive effect by extract of Mentharo tundifolia leaves on the corrosion of steel in 1 M HCl. solution. Res. Chem. Intermed. 40, 961–972 (2014)
B.P. Markhali, R. Naderi, M. Mahdavian, M. Sayebani, S.Y. Arman, Electrochemical impedance spectroscopy and electrochemical noise measurements as tools to evaluate corrosion inhibition of azole compounds on stainless steel in acidic media. Corr. Sci. 75, 269–279 (2013)
C.B. Pradeep Kumar, K.N. Mohana, Adsorption and thermodynamic characteristics of plumeriarubra plant extracts on mild steel corrosion in industrial water medium. Int. Res. J. Pure Appl. Chem. 3, 330–346 (2013)
H. Jafari, I. Danaee, H. Eskandari, M.R. Avei, Electrochemical and theoretical studies of adsorption and corrosion inhibition of N,N′-bis(2-hydroxyethoxyacetophenone)-2,2-dimethyl-1,2-propanediimine on low carbon steel (API 5L Grade B) in acidic solution. Ind. Eng. Chem. Res. 52, 6617–6632 (2013)
A. Popova, S. Raicheva, E. Sokolova, M. Christov, Frequency dispersion of the interfacial impedance at mild steel corrosion in acid media in the presence of benzimidazole derivatives. Langmuir 12, 2083–2089 (1996)
M. Lebrini, M. Lagrenee, H. Vezin, M. Traisnel, F. Bentiss, Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corros. Sci. 49, 2254–2269 (2009)
A.S. El-Tabei, M.A. Hegazy, Corrosion inhibition study of a novel synthesized gemini nonionic surfactant for carbon steel in 1 M HCl solution. J. Surfact. Deterg. 16, 757–766 (2013)
P. Singh, A. Singh, M.A. Quraishi, S. El-Tabei, M.A. Hegazy, A inhibition effect of 1,3,5-tri-p-tolyl-1,3,5-triazene on the corrosion of brass in 0.5 M HCl solution. Res. Chem. Intermed. 40, 595–604 (2014)
M. Abdallah, Rhodanineazosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 44, 717–728 (2002)
O. Ghasemi, I. Danaee, G.R. Rashed, R.M. Avei, M.H. Maddahy, Inhibition effect of a synthesized N,N′-bis(2-hydroxybenzaldehyde)-1,3-propandiimine on corrosion of mild steel in HCl. J. Centr. South Univ. 20, 301–311 (2013)
P. NarayanaHebbar, B.M. Prasanna, T.V. Venkatesha, Corrosion inhibition behavior of ketosulphidefor mild steel in acidic medium. Int. Res. J. Chem. 2, 018–020 (2015)
S.E. Nataraja, T.V. Venkatesha, H.C. Tandon, Computational and experimental … inhibition of steel by tacrine. Corros. Sci. 60, 214–223 (2012)
H.Z. AL-Sawaad, Evaluation of the ceftriaxone as corrosion inhibitor for carbon steel alloy in 0.5 M of hydrochloric acid. Int. J. Electron. Sci. 8, 3105–3120 (2013)
B. Obot, N.O. Obi-Egbedi, A.O. Eseola, Anticorrosion potential of 2-mesityl-1H-imidazo[4,5-f][1,10] phenanthroline on mild steel in sulfuric acid solution: experimental and theoretical study. Ind. Eng. Chem. Res. 50, 2098–2110 (2011)
R.S.A. El-Hameed, Aminolysis of polyethylene terephthalate waste as corrosion inhibitor for carbon steel in HCl corrosive medium. Adv. Appl. Sci. Res. 2, 483–499 (2011)
A.K. Singh, M.A. Quraishi, The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acid. Corros. Sci. 52, 1373–1385 (2010)
N. Hebbar, B.M. Praveen, B.M. Prasanna, T.V. Venkatarangaiah, The Corrosion inhibition effect of hydralazine. HCl on the zinc in acidic media. Mor. J. Chem. 3, 496–506 (2015)
M. Mobin, M.A. Khan, B.M. Parveen, Inhibition of mild steel corrosion in acidic medium using starch and surfactants additives. J. Appl. Polym. Sci. 121, 1558–1565 (2011)
K. Pavithra, T.V. Venkatesha, K. Vathsala, K.O. Nayana, Synergistic effect of halide ions on improving corrosion inhibition behavior of benzisothiozole-3-piperizine hydrochloride on mild steel in 0.5 M H2SO4 medium. Corr. Sci. 52, 3811–3819 (2010)
H. Ashassi-Sorkhabi, B. Masoumi, P. Ejbari, E. Asghari, Corrosion inhibition of mild steel in acidic media by basic yellow-13 dye. J. Appl. Electrochem. 39, 1497–1501 (2009)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hebbar, N., Praveen, B.M., Prasanna, B.M. et al. Electrochemical and Adsorption Studies of 4-Chloro,8-(Trifluoromethyl)Quinoline (CTQ) for Mild Steel in Acidic Medium. J Fail. Anal. and Preven. 20, 1516–1523 (2020). https://doi.org/10.1007/s11668-020-00944-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-020-00944-4