Abstract
Present study discusses about a technique for producing high-purity Cr2AlC MAX phase materials and gaining insight into their thermal behavior for high-temperature applications. The research conducted involved synthesizing a pure layered ternary carbide Cr2AlC MAX phase material by mixing powders of Chromium, Aluminum, and Carbon and then subjecting them to two-step pressureless sintering process in argon atmosphere. First step involves the annealing of ball-milled mixture at 750 °C for 2 h followed by the second step in which the annealed mixture is subjected to heat-treatment at 1350 °C for 2 h. Analysis using XRD and Raman techniques revealed that the synthesized product consists of Cr2AlC phase, without any impurities. SEM studies confirmed that the Cr2AlC had a layered topography, while EPMA analysis indicated that the atomic percentage of Cr, Al, and C was consistent with the XRD phase analysis. XPS investigations confirmed the presence of Cr-C bonds representing Mn+1Xn of the MAX phase material. TG-DSC results showed an approximately 2% increase in weight. The Cr2AlC phase exhibited an endothermic pattern below 725 °C, an exothermic pattern above it, and did not decompose up to 1400 °C in vacuum environment. High-temperature XRD analysis at various temperatures also confirmed no formation of Al2O3 or CrO impurity compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MAX phase materials are a unique category of materials that exhibit a distinctive combination of metallic and ceramic characteristics. They consist of ternary layered nitrides and carbides with a general chemical formula of Mn+1AXn, where M denotes an early transition metal, A represents a predominantly IIIA- or IVA-group element, and X can be either carbon or nitrogen (Ref 1). These MAX phases possess a hexagonal crystal structure and belong to the P63/mmc space group (Ref 2). Noteworthy properties of MAX phase materials include high thermal conductivity, exceptional strength, excellent machinability, strong resistance to corrosion, high temperature oxidation resistance, low density, and low thermal expansion coefficient (Ref 3,4,5,6,7). Currently, a combination of theoretical and experimental studies has documented approximately 150 distinct compositions of MAX phases (Ref 8).
Cr2AlC belongs to the MAX phase family of materials, which was initially discovered by Jeitschko et al. (Ref 9) in 1963 and later confirmed by Schuster et al., in 1980 (Ref 10). It possess several notable properties, including high elastic modulus (Ref 7), excellent corrosion resistance against Na2SO4 at approximately 1000 °C (Ref 7), excellent oxidation resistance up to 1300 °C (Ref 7), excellent radiation resistance (Ref 11), good mechanical properties at room temperature (Ref 12). Additionally, Tian et al, reported that Cr2AlC exhibits good electrical conductivity ranging from 1.4 to 2.3 × 10−6 Ω−1 m−1 and thermal conductivity ranging from 17.5 to 22.5 W/m K (Ref 13). It has been observed that the corrosion and oxidation resistance of Cr2AlC surpasses that of Ti3AlC2 and Ti3SiC2 (Ref 7, 14). These properties render Cr2AlC suitable for various applications involving graphite-metal composites, such as electrical plates, pantographs, bipolar plates in fuel cells, and photocatalysis, among others (Ref 8, 15). Furthermore, its high temperature oxidation resistance makes it suitable for use in the nuclear industry, structural applications, and gas burner nozzles (Ref 16).
Various methods have been employed for the synthesis of Cr2AlC including Hot pressing (HP) (Ref 17), spark plasma sintering (SPS) (Ref 18), pressureless sintering, molten salt method (Ref 19) and hot isostatic pressing (HIP) (Ref 20). Manoun et al., achieved successful synthesis of Cr2AlC by mixing of Cr, Al, and C, utilizing HIP method at a temperature of 1200 °C and an approximate pressure of 100 MPa (Ref 20). Conversely, Yukhvid et al. prepared Cr2AlC using precursors such as Cr2O3, CrO3, Al, and C through the self-propagating high-temperature synthesis (SHS) method under an argon atmosphere at a pressure of 5 MPa (Ref 21). Additionally, Zhou et al. investigated the reaction kinetics and mechanical properties of Cr2AlC synthesized via the hot pressing technique at 1300 °C and at 30 MPa pressure (Ref 22). Cr2AlC were synthesized by Li et al, at relatively lower temperature at 1100 °C and 30 MPa using Hot Pressing (Ref 17). However, it is important to note that these processes typically require elevated temperatures exceeding 1000 °C, high pressures of up to 100 MPa, and complex equipment, which restricts their widespread applicability (Ref 23).
The production of high-quality Cr2AlC powder presents a significant challenge due to various factors discussed in previous studies, including the low melting point of Al above 660 °C, the intense exothermal interaction between Cr and C, and the degradation of Cr2AlC beyond 1400 °C (Ref 17, 24). Despite considerable advancements in this area, there remains a gap in achieving the synthesis of Cr2AlC with high purity, as impurities such as Cr7C3 and Cr2Al phases are often observed alongside Cr2AlC (Ref 25, 26). While techniques like hot pressing, hot isostatic pressing, and spark plasma sintering have been explored, their cost-effectiveness makes them impractical for continuous or bulk manufacturing. However, the pressureless sintering method provides an affordable and excellent option for mass production, enabling the creation of samples with complex and large shapes. Julian et al, reported less degradation of Cr2AlC when employing pressureless sintering (Ref 27). Panigraphi et al. reported the synthesis of Cr2AlC using pressureless sintering by initially forming partially sintered CrCx powder and subsequently mixing it with Al (Ref 28). The production of CrCx powder occurred at 1550 °C, followed by a mixing with Al, and subsequent furnace treatment to create Cr2AlC at 1100 °C. This sequential procedure increases both the cost and time required for Cr2AlC synthesis. A review of the literature revealed the necessity of achieving pure Cr2AlC using pressureless sintering. The key challenges involve selecting the appropriate initial materials, potential formation of intermediate compounds during synthesis, Al oxidation during the reaction, the two-step sequential process, and the presence of impurities in the final Cr2AlC product. Choi et al. and Tian et al. emphasized the importance of forming Cr2Al in the synthesis of Cr2AlC (Ref 24, 25). This paper introduces a novel two-step reaction strategy to achieve the synthesis of ultra-pure Cr2AlC through pressureless sintering under an inert atmosphere. The method involves the initial formation of intermetallic Cr-Al compounds at 750 °C, followed by the subsequent reaction of these intermetallics with graphite to produce Cr2AlC at 1350 °C.
MAX phases demonstrate exceptional suitability for high-temperature applications, emphasizing the significance of their oxidation and heat resistance properties. The stability of these materials under elevated temperatures stands as a pivotal subject for investigation. The remarkable oxidation resistance of Cr2AlC has also been widely recognized. However, MAX phases, including Cr2AlC, have a tendency to decompose at extremely high temperatures. For instance, Ti3SiC2 becomes unstable at temperatures exceeding 1400 °C in a vacuum or argon atmosphere (Ref 29,30,31), while Ti3AlC2 decomposes above 1400 °C in a vacuum environment (Ref 32, 33). In contrast, according to Xiao et al., Cr2AlC demonstrates thermal stability in an argon atmosphere remaining stable up to 1500 °C (Ref 16). Lee at al. investigated the oxidation of Cr2AlC in air upto 1200 °C (Ref 34), but information regarding the of thermal stability of Cr2AlC in vacuum environment are scarce.
This study aims to establish a cost-effective synthesis method for obtaining pure Cr2AlC without impurities. Additionally, it seeks to assess the thermal stability of Cr2AlC in a vacuum environment by employing Differential Scanning Calorimetry and High Temperature x-ray Diffraction techniques.
2 Experimental Details
2.1 Synthesis of Cr2AlC
The starting materials used to synthesize Cr2AlC were powders of Chromium (≥ 99% trace metal basis, − 325 mesh, Sigma‐Aldrich, St. Louis, MO), Al (99.5% trace metal basis, − 325 mesh, Alfa Aesar, Haverhill, MA), and graphite (Graphite powder crystalline, 99%, − 325 mesh, Alfa Aesar, Haverhill, MA). These precursors, viz. Cr, Al, and C were uniformly mixed using dry ball milling (8000 M mixer Mill; SPEX SamplePrep) in a molar ratio of 2:1.1:1, respectively. Similar molar ratios were already used for successful synthesis of Cr2AlC by Hall et al. (Ref 35). Subsequently, the ball-milled powder was cold-pressed in to small pellets. These pellets are transferred into a tube furnace in which a continuously flowing Ar environment is maintained. These pellets are exposed to a thermal process cycle as shown in Fig. 1. The temperature inside the furnace was increased at a rate of 10 °C/min up to 750 °C, and the pellets were annealed for 2 h at this temperature. Then in the second step, the rate of heating was set at 5 °C/min till the heat treatment temperature i.e., 1350 °C is reached. At this temperature the heat treatment was carried out for 2 h. Then, the furnace was switched off and the pellets were cooled to room temperature through furnace cooling. A positive pressure of 1.1 bar is always maintained in the tube furnace in order to prevent the oxidation of Aluminum.
2.2 Characterization Techniques
The synthesized Cr2AlC powders were characterized using various techniques including x-ray Diffraction (XRD), scanning electron microscopy (SEM), Electron Probe Micro analyzer (EPMA), Raman spectroscopy, and XPS. Cr2AlC exhibits excellent oxidation resistance, and to understand its thermal stability at high temperatures in sub-atmospheric pressure, an assessment study was conducted using Differential Scanning Calorimetry and High-temperature XRD.
To identify the phases that are present in the powder, Cr2AlC was subjected to XRD analysis using an XRD equipment (model: Bruker D8 discover, Supplier: Bruker GmbH, Germany) with Cu Kα radiation (λ = 1.5406 Å) in the 2θ range of 20-90° with a step size of 0.02° Bragg-Brentano mode. The crystallite size was computed using the Scherrer formula and the instrumental broadening was minimized by comparing the observed sample peak width (FWHM) to that of a typical reference sample. To investigate the thermal stability of Cr2AlC, High temperature XRD was done using the Anton Par heating attachment in the same equipment. The High Temperature XRD was carried out in vacuum with a working pressure of 5 × 10−2 mbar to study the stability of Cr2AlC and investigate whether that reversible phase transition occurred at high temperature. The base pressure of the HT-XRD system was 1 × 10−6 mbar. The step size for HT-XRD was 0.050, scan speed of 1 s/step and the ramp rate of 10 °C/min. The tube voltage was kept at 40 kV and tube current at 30 mA. The calibration of the high-temperature x-ray diffraction (HT-XRD) system involved the use of MgO as a standard reference material. This meticulous calibration process aimed to establish accurate relationships between the observed diffraction patterns and known characteristics of MgO, providing a reliable foundation for temperature-dependent XRD analyses. The Z axis calibration was also carried using MgO as standard material at every temperature cycle. Consideration of the thermal response of the applied thermocouple is also important for the evaluation of the calibration results. The S-type Pt/Pt90- Rh10 thermocouple used in this study responds linear in the temperature range from approx. 350 up to 1200 °C.
Raman spectroscopy was performed to examine the vibrational, rotational and sample’s structural characteristics with a confocal microscope (Model: Qontor by Renishaw). The spectrometer has a wavelength of 532 nm (He-Ne laser) and diffraction grating of 1200 grooves/mm.
The morphology of Cr2AlC was studied using High resolution scanning electron microscopy (HRSEM; Model: Carl Zeiss, Supplier: VP Merlin, Germany) and its elemental composition was found out using electron probe micro analyzer (EPMA).
Cr2AlC was studied using x-ray photoelectron spectroscopy (Model: Thermo-Scientific NEXSA) to determine its chemical states and bond energies. An excitation source employed was Al kα radiation with an energy of 1486.6 eV. The sample surface was sputter cleaned using an Ar ion beam at 3 keV for 5 min. The C1s peak, which corresponds to a binding energy of 284.6 eV, was used to calibrate the binding energy scale.
To determine the temperature-dependent phase changes, TG-DSC (Thermogravimetric and differential scanning calorimetry) study was carried out by a simultaneous thermal analyser (Model: Linseis GmbH, STA PT 1600). In the fused alumina crucibles, experiments on Cr2AlC powder samples were carried out up to 1400 °C in vacuum at a pressure of 5 × 10−2 mbar. In the fused alumina crucibles, experiments on Cr2AlC powder samples were carried out up to 1400 °C. The system’s drift was assessed, and the results of the experiment were subtracted. The heating rate for the dynamic run was set to 10 °C per minute. The sample temperature was monitored using a type S thermocouple.
3 Results and Discussion
3.1 Reaction Mechanism
Figure 1 illustrates the process cycle employed in the synthesis of Cr2AlC. As it is clear from this figure, the present pressureless sintering process consists of two main steps to facilitate the Cr-Al formation. (i) In the first step, the mixture of chromium, aluminum, and carbon is heated up to 750 °C and then annealed at this temperature for 2 h. This annealing temperature was chosen to be just above melting point of Aluminum, because of the following reason. At this temperature, Al will be in the molten state and the reaction rate between liquid Al and solid Cr would be much higher than that of between solid Al and solid Cr. As this annealing temperature is kept constant for sufficient time (2 h), the liquid aluminum selectively reacts with some chromium powders, either bonding with individual chromium atoms or forming chromium aluminides such as Cr2Al and Cr5Al8. The carbon remains unreacted at this annealing temperatures. During the second step i.e., heat treatment at a high temperature of 1350 °C, the chromium-aluminum system undergoes complete reaction with carbon, resulting in the formation of Cr2AlC. The synthesized product is furnace cooled to room temperature. The excess Al used in the process (1.1 moles instead of 1 mole), was required in order to compensate for any possible losses of Al that could arise during step 1 of the synthesis. The synthesized and cooled sample was then ground into very fine size with the help of agate mortar-pestle and sieved (325 mesh size), for further characterization.
3.2 X-ray Diffraction
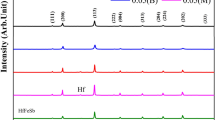
The XRD spectra of Cr2AlC synthesized at 1350 °C are shown in Fig. 2. The spectra revealed (103), (006), and (109) reflections that belong to the Cr2AlC phase, as per ICDD file number 00-058-0267. A high-resolution scan was performed between 2θ values of 40° and 45°, in order to distinguish the very close peaks at 42.0716° and 42.1784° and both of these represent Cr2AlC. The high-resolution XRD scan is shown in Fig. 3. Absence of peaks representing pure elements like Cr, Al, etc. or impurity compounds such as Cr7C3 Cr2Al and Al2O3, in the XRD results, indicates about the synthesis of pure Cr2AlC. Rietveld refinement were performed on the synthesized Cr2AlC using Full PROF suite (Ref 36) and the lattice parameters of this layered Cr2AlC were calculated, and the values for a, b, and c were found to be 2.862, 2.862, and 12.838 Å, respectively, which aligns with the values in the ICDD database file. The crystallite size was calculated using Scherer’s formula, and it was found to be around 102 nm.
3.3 Raman Spectroscopy
To confirm the single-phase synthesis of Cr2AlC at 1350 °C as identified by XRD, Raman spectroscopy was employed to analyze its vibration bands. As shown in Fig. 4, all of the peaks observed in the Raman spectra of Cr2AlC correspond to its vibrational bands. The MAX phases are classified under the D46h space group and Raman signals are given in Table 1. The four Raman active optical modes belong to the 211 structures, three of which are Raman active i.e., 153.43, 245.53, 249.12 cm−1 and the one that is 335.74 cm−1 is both Raman and infrared active. (Ref 37,38,39). The wave numbers of the Raman spectra are given in Table 1, and they are in alignment with the theoretical data.
3.4 SEM and EPMA
SEM was used for studying the morphology of the synthesized Cr2AlC. The powder sample for SEM analysis was collected and sieved (325 mesh) to ensure uniformity, then mounted on a conductive substrate using carbon tape, followed by careful handling to prevent contamination. A conductive coating was not deemed necessary as Cr2AlC is conductive in nature. SEM micrographs captured at various magnifications are shown in Fig. 5. It is clear from the SEM images that the synthesized Cr2AlC is having a layered structure, and the average particle size is approximately 45 microns. During SEM studies, random EDS analysis of Cr2AlC was performed and the results indicate that the average atomic percent of Cr is approximately 52.99%, whereas Carbon is approx. 24.19% and Al approx. 22.81%. EPMA studies were also performed for determining the elemental composition of Cr2AlC. Point scans were conducted at five different locations and the results of the same are given in Table 2. The same results are shown in graphical form in Fig. 6. It is clear from these results that the elemental composition is in line with the stoichiometry of Cr2AlC. These results are complimenting the XRD results.
3.5 X-ray Photoelectron Spectroscopy
Cr2AlC, confirmed by x-ray diffraction (XRD) and Raman spectroscopy, was analyzed further using x-ray photoelectron spectroscopy (XPS) to gain insight into the bonding characteristics of this MAX phase material. The XPS core level binding energy spectra of Cr2AlC were fitted using XPSPEAK 4.1 software. Figure 7 shows the fitted core level energy spectra for Cr2p, Al2p, C1s, and O1s of Cr2AlC. As it can be seen from the results, the XPS analysis revealed the presence of oxygen. The reason for the presence of Oxygen is likely due to prolonged exposure of the synthesized powder to air, could have lead to the formation of Cr and Al oxides. However, the absence of any oxide peaks in XRD, and Oxygen in EPMA results indicates that these oxides must have formed as thin layers on the surface only. Abedlkader reported that selective oxidation of Cr and Al occurs on the surface of Cr2AlC powder in air (Ref 40). After deconvolution, Cr2p spectra revealed four peaks i.e., Cr-C bond peaks at binding energy 573.5 and 583.4 eV. Similar observations were made by Shtansky et al. and Zhang et al.(Ref 41, 42) and Cr-O bond peaks at binding energy 576.18 and 586.9 eV corresponding to Cr2O3 which is in sync with observations by Sleight and Agostinelli (Ref 43, 44). Similarly, after deconvolution the Al 2p spectra revealed two peaks: Al-O bond peaks at binding energies of 71.25 and 74.3 eV corresponding to Al2O3, the similar peak positions were observed by Gillet et al. and Ertl et al. (Ref 45, 46). The aluminum (Al) component within Cr2AlC forms a bond characterized by a weak electrostatic force, rendering it susceptible to oxidation. This tendency of Al to oxidize is in alignment with the findings presented by Naslund et al. and Desai et al., who have noted the weak electrostatic nature of Al bonds in MAX phases containing aluminum (Ref 47, 48). Furthermore, the work of Baben et al. highlights instances of oxygen incorporation in M2AlC. (Ref 49). After deconvolution, the C1s spectra showed four peaks located at 282.1, 284.6, 285.9 and 288.6 eV, respectively. The peak observed at 282.1 can be attributed to the typical carbon valence state for carbon that is carbide-like i.e., Cr-C as reported by Shtansky et al. (Ref 41). The peaks located at 284.6 and 285.94 eV corresponds to basic C-C bond. Identical observations were observed by Abdelkader et al. and Moncoffre et al. (Ref 40, 50). Additionally, the presence of C-O bond is detected at the peak located at 288.6 eV in line with the observation of Moncoffre, N et al. (Ref 50). Although oxygen is not inherently present in Cr2AlC; the samples were exposed to laboratory atmosphere condition and hence, the possibility of O being present in the form of Cr2O3 as well as Al2O3 needs to be further investigated. The O1s spectra were deconvoluted, revealing two peaks at 529.9 and 531.7 eV, corresponding to the Cr-O bond of Cr2O3 and the Al-O bond of Al2O3, Stypula, B, and Stypula, B et al. have reported comparable binding energies associated with Cr2O3 and Al2O3 (Ref 51, 52).
3.6 High Temperature XRD
High Temperature x-ray diffraction studies were performed for investigating the thermal stability of synthesized Cr2AlC. If the Al is weakly bonded in the MAX phase material, it could get oxidized as the temperature increases. Further, it could also get evaporated at the prevailing temperatures of the high temperature XRD test, as this test is conducted at sub-atmospheric pressures, viz. around 5 × 10−2 mbar. The results of this high temperature XRD tests – performed at various temperatures ranging from room temperature to 1000 °C – are shown in Fig. 8. From the results, it is observed that the peak positions of Cr2AlC slightly shifted with at higher temperatures. Possible reason for this finding may be because of thermal expansion. Further it is also observed that the intensities of Cr2AlC peaks decreased with increasing temperatures. However, there is no indication on the formation of Al2O3 or any other oxide or any other impurity, with the increase in temperature up to 1000 °C. These results confirm about the phase stability of the synthesized Cr2AlC up to 1000 °C, in the sub-atmospheric low pressure conditions.
3.7 TG-DSC
The synthesized Cr2AlC was subjected to TG-DSC studies also, in order to analyze its thermal stability. The tests were performed at sub-atmospheric pressure conditions, viz. at around 5 × 10−2 mbar and the temperature was increased up to 1400 °C. The results of TG-DSC studies are shown in Fig. 9. The TG curve indicates that the rate of oxidation increases in direct correlation to the temperature of the test. Further, it has also revealed that there is an approximate 2% weight gain in the sample after TG test, possibility of surface oxidation of the sample. On the other hand, the DSC curve reveals that below 785 °C the primary reaction was endothermic, attributed to sample heating. However, above 785 °C the dominant reaction became exothermic, indicating the oxidation of Cr2AlC. It is the inherent property of Cr2AlC to form a continuous protective surface layer of Al2O3 when subjected to high temperature (Ref 8). Consequently, noticeable oxidation of Cr2AlC commences above 785 °C (Ref 53). Whenever Cr2AlC underwent oxidation, it produces a layer of Al2O3 on the surface. In addition, the consumption of Al during this process causes the formation of a layer of Cr7C3 underneath the Al2O3 layer. This layer of Cr7C3 is known for its hardness and is stabilized by strong interactions between Cr and C atoms (Ref 53).
Samples, after TG-DSC studies, were subjected again to XRD analysis in order to investigate the new phases that might have formed during TG test which must have contributed to increase in weight. They were also analyzed with SEM to check for any morphological changes occurred during TG-DSC test. The x-ray diffraction (XRD) results are shown in Fig. 10. The primary peaks are identified with Cr2AlC phase, while additional peaks representing Al2O3 (ICDD 00-001-1296) and Cr7C3 (03-065-1347) were also detected. SEM results also complemented these findings. SEM micrographs, shown in Fig. 11, clearly indicate the appearance of flower-like morphology, which is associated with α-Al2O3 (Ref 50); along with regular Cr2AlC. SEM energy-dispersive spectroscopy (EDS) analysis was conducted at a few points, and the results are shown in Table 3. EDS results of P4, P5 and P6 data points substantiate the coexistence of Al2O3 and Cr2AlC. This particular oxidation of aluminum primarily occurred on the surface, potentially resulting in the transformation of Cr2AlC into Cr3C7 and Al2O3 in the top layers. The oxidation of aluminum in Cr2AlC can be attributed to two factors: its higher affinity for oxygen when compared to chromium and carbon, and the greater Gibbs free energy for the reaction between aluminum and oxygen compared to other elements such as chromium and carbon.
4 Conclusions
A new two-step pressureless sintering process was successfully employed for producing high purity Cr2AlC powders using commercially available Cr, Al, and C powders. Liquid-solid reactions between Al and Cr, respectively, in the first step were used for the formation of Chromium Aluminide intermetallics. A full reaction between these intermetallics and Carbon is ensured in the second step (at 1350 °C). Formation of pure Cr2AlC is confirmed through x-ray Diffraction and Raman spectroscopy analyses. Scanning electron microscopy (SEM) results provided evidence on the existence of layered morphology of Cr2AlC grains; while the EPMA quantitative elemental analysis resulted in atomic percentages of 52.99% Cr, 22.81% Al, and 24.19% C and are found to be aligning with XRD findings.
Additional insights from x-ray Photoelectron Spectroscopy (XPS) investigations unveiled the bonding nature, with Cr-C bonding being identified. HT-XRD studies, conducted at various temperatures, confirmed that there is no oxide formation up to 1000 °C indicating of the Cr2AlC phase stability. However, Differential scanning calorimetry (DSC) data demonstrated the onset of oxide formation after 785 °C, and thermogravimetric analysis (TG) revealed an approximate 2% increase in weight, corroborating the occurrence of oxidation. XRD and SEM (EDS) analysis of post TG-DSC samples confirmed the presence of Al2O3. This is leading us to conclude that there is oxide formation with increase in temperature but only through a few layers on the surface and is not in considerable quantity to be identified in HT-XRD up to 1000 °C. Oxidation is relatively higher during TG-DSC tests as they were conducted up to 1400 °C, and the same was reflected through increase in weight by approximately 2% and presence of oxide peaks in XRD results of post TG-DSC samples.
These comprehensive analyses collectively affirm the successful synthesis of Cr2AlC powders with high purity and reveal valuable insights into the fundamental properties of the material.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
M.W. Barsoum, The MN+ 1AXN Phases: A New Class of Solids: Thermodynamically Stable Nanolaminates, Prog. Solid State Chem., 2000, 28(1–4), p 201–281.
M.W. Barsoum and M. Radovic, Elastic and Mechanical Properties of the MAX Phases, Annu. Rev. Mater. Res., 2011, 41, p 195–227.
X. He et al., General Trends in the Structural, Electronic and elastic Properties of the M3AlC2 Phases (M= transition metal): A First-Principle Study, Comput. Mater. Sci., 2010, 49(3), p 691–698.
M.W. Barsoum, D. Brodkin, and T. El-Raghy, Layered Machinable Ceramics for High Temperature Applications, Scripta Mater., 1997, 36(5), p 535–541.
T. Scabarozi et al., Thermal Expansion of Select Mn+1AXn (M = Early Transition Metal, A = A Group Element, X = C or N) Phases Measured by High Temperature X-ray Diffraction and Dilatometry, J. Appl. Phys., 2009, 105(1), 013543.
M. Radovic and M.W. Barsoum, MAX Phases: Bridging the Gap Between Metals and Ceramics, Am. Ceram. Soc. Bull., 2013, 92(3), p 20–27.
Z. Lin et al., High-Temperature Oxidation and Hot Corrosion of Cr2AlC, Acta Mater., 2007, 55(18), p 6182–6191.
J. Gonzalez-Julian, Processing of MAX Phases: From Synthesis to Applications, J. Am. Ceram. Soc., 2021, 104(2), p 659–690.
W. Jeitschko, H. Nowotny, and F. Benesovsky, Kohlenstoffhaltige ternäre Verbindungen (H-phase), Monatshef. Chem. Verwandte Teile anderer Wissenschaften, 1963, 94(4), p 672–676.
J. Schuster, H. Nowotny, and C. Vaccaro, The Ternary Systems: CrAlC, VAlC, and TiAlC and the Behavior of H-Phases (M2AlC), J. Solid State Chem., 1980, 32(2), p 213–219.
M. Imtyazuddin, A.H. Mir, M.A. Tunes, and V.M. Vishnyakov, Radiation Resistance and Mechanical Properties of Magnetron-Sputtered Cr2AlC Thin Films, J. Nucl. Mater., 2019, 526, p 151742. https://doi.org/10.1016/j.jnucmat.2019.151742
W. Tian et al., Mechanical Properties of Pulse Discharge Sintered Cr2AlC at 25–1000° C, Mater. Lett., 2009, 63(8), p 670–672.
W. Tian et al., Synthesis and Thermal and Electrical Properties of Bulk Cr2AlC, Scripta Mater., 2006, 54(5), p 841–846.
D. Lee and T.D. Nguyen, Cyclic Oxidation of Cr2AlC Between 1000 and 1300° C in Air, J. Alloys Compd., 2008, 464(1–2), p 434–439.
B.S. Reghunath, D. Davis, and K.S. Devi, Synthesis and Characterization of Cr2AlC MAX Phase for Photocatalytic Applications, Chemosphere, 2021, 283, 131281.
L.-O. Xiao et al., Synthesis and Thermal Stability of Cr2AlC, J. Eur. Ceram. Soc., 2011, 31(8), p 1497–1502.
S. Li et al., Mechanical Properties of Low Temperature Synthesized Dense and Fine-grained Cr2AlC Ceramics, J. Eur. Ceram. Soc., 2011, 31(1–2), p 217–224.
W. Tian et al., Synthesis Reactions of Cr2AlC from Cr-Al4C3-C by Pulse Discharge Sintering, Mater. Lett., 2008, 62(23), p 3852–3855.
W.-B. Tian, P.-L. Wang, Y.-M. Kan, and G.-Jn. Zhang, Cr2AlC Powders Prepared by Molten Salt Method, J. Alloys Compd., 2008, 461(1–2), p L5–L10. https://doi.org/10.1016/j.jallcom.2007.06.094
B. Manoun et al., Compression Behavior of M2Al C (M= Ti, V, Cr, Nb, and Ta) Phases to Above 50 GPa, Phys. Rev. B, 2006, 73(2), 024110.
V. Gorshkov et al., SHS Metallurgy of Cr2AlC MAX Phase-Based Cast Materials, Russ. J. Non-Ferrous Met., 2018, 59(5), p 570–575.
M. Yan et al., Effect of Ball Milling Treatment on the Microstructures and Properties of Cr2AlC Powders and Hot Pressed Bulk Ceramics, J. Eur. Ceram. Soc., 2019, 39(16), p 5140–5148.
Z. Lin et al., In-situ Hot Pressing/Solid-Liquid Reaction Synthesis of Bulk Cr2AlC, Int. J. Mater. Res., 2005, 96(3), p 291–296.
H.-C. Oh, S.-H. Lee, and S.-C. Choi, The Reaction Mechanism for the Low Temperature Synthesis of Cr2AlC under Electronic Field, J. Alloys Compd., 2014, 587, p 296–302.
W.-B. Tian et al., Phase Formation Sequence of Cr2AlC Ceramics Starting from Cr-Al-C Powders, Mater. Sci. Eng.A, 2007, 443(1–2), p 229–234.
G. Ying et al., Synthesis and Mechanical Properties of High-Purity Cr2AlC Ceramic, Mater. Sci. Eng., 2011, 528(6), p 2635–2640.
J. Gonzalez-Julian et al., Effect of Sintering Method on the Microstructure of Pure Cr2AlC MAX Phase Ceramics, J. Ceram. Soc. Jpn., 2016, 124(4), p 415–420.
B.B. Panigrahi et al., Reaction Synthesis and Pressureless Sintering of Cr2AlC Powder, J. Am. Ceram. Soc., 2010, 93(6), p 1530–1533.
I.M. Low, Z. Oo, and K. Prince, Effect of Vacuum Annealing on the Phase Stability of Ti3SiC2, J. Am. Ceram. Soc., 2007, 90(8), p 2610–2614.
C. Racault, F. Langlais, and R. Naslain, Solid-State Synthesis and Characterization of the Ternary Phase Ti3SiC2, J. Mater. Sci., 1994, 29(13), p 3384–3392.
Z. Oo, I. Low, and B. O’Connor, Dynamic Study of the Thermal Stability of Impure Ti3SiC2 in Argon and Air by Neutron Diffraction, Phys. B, 2006, 385, p 499–501.
S.-B. Li et al., Synthesis and Microstructure of Ti3AlC2 by Mechanically Activated Sintering of Elemental Powders, Ceram. Int., 2007, 33(2), p 169–173.
W.K. Pang, I.M. Low, and Z.M. Sun, In situ High-Temperature Diffraction Study of the Thermal Dissociation of Ti3AlC2 in Vacuum, J. Am. Ceram. Soc., 2010, 93(9), p 2871–2876.
D. Lee and S. Park, Oxidation of Cr2AlC Between 900 and 1200 °C in Air, Oxid. Met., 2007, 68(5), p 211–222.
K. Hall et al., Synthesis and Characterization of Novel Polymer Matrix Composites Reinforced with Max Phases (Ti3SiC2, Ti3AlC2, and Cr2AlC) or MoAlB by Fused Deposition Modeling, Int. J. Ceram. Eng., 2019, 1(3), p 144–154.
K.Y. Karuna et al., Nanocrystalline Cr2AlC-MAX Phase Formation During Mechanically Activated Annealing of Al-31Cr-7C, Trans. Indian Inst. Met, 2021, 74(9), p 2313–2318. https://doi.org/10.1007/s12666-021-02324-4
J.E. Spanier et al., Vibrational Behavior of the Mn+1AXn Phases from First-order Raman Scattering (M = Ti, V, Cr, A = Si, X = C, N), Phys. Rev. B, 2005, 71(1), 012103.
O.D. Leaffer et al., On Raman Scattering from Selected M2AC Compounds, J. Mater. Res., 2007, 22(10), p 2651–2654.
J. Wang et al., Raman Active Phonon Modes and Heat Capacities of Ti2AlC and Cr2AlC Ceramics: First-Principles and Experimental Investigations, Appl. Phys. Lett., 2005, 86(10), 101902.
A.M.J. Abdelkader, Molten Salts Electrochemical Synthesis of Cr2AlC, J. Eur. Ceram. Soc., 2016, 36(1), p 33–42.
D. Shtansky et al., Comparative Investigation of TiAlC (N), TiCrAlC (N), and CrAlC (N) Coatings Deposited by Sputtering of MAX-Phase Ti2−xCrxAlC Targets, Surf. Coat. Technol., 2009, 203(23), p 3595–3609.
Y. Zhang et al., High Antioxidant Lamellar Structure Cr2AlC: Dielectric and Microwave Absorption Properties in X Band, J. Alloys Compd., 2021, 860, 157896.
C. Sleigh et al., On the Determination of Atomic Charge Via ESCA Including Application to Organometallics, J. Electron Spectrosc. Relat. phenom., 1996, 77(1), p 41–57.
E. Agostinelli et al., An XPS Study of the Electronic Structure of the ZnxCd1−xCr2 (X = S, Se) Spinel System, J. Phys., 1989, 50(3), p 269–272.
E. Gillet and B. Ealet, Characterization of Sapphire Surfaces by Electron Energy-Loss Spectroscopy, Surf. Sci., 1992, 273(3), p 427–436.
G. Ertl et al., XPS Study of Copper Aluminate Catalysts, Appl. Surf. Sci., 1980, 5(1), p 49–64.
V. Desai et al., Pressureless Manufacturing of High Purity Ti3AlC2 MAX Phase Material: Synthesis and Characterisation, Vacuum, 2023, 214, p 112221. https://doi.org/10.1016/j.vacuum.2023.112221
L.-Å. Näslund, P.O.Å. Persson, and J. Rosen, X-ray Photoelectron Spectroscopy of Ti3AlC2, Ti3C2Tz, and TiC Provides Evidence for the Electrostatic Interaction between Laminated Layers in MAX-Phase Materials, J. Phys. Chem. C, 2020, 124(50), p 27732–27742. https://doi.org/10.1021/acs.jpcc.0c07413
M. Baben et al., Oxygen Incorporation in M2AlC (M = Ti, V, Cr), Acta Mater., 2012, 60(12), p 4810–4818.
N. Moncoffre et al., Temperature Influence During Nitrogen Implantation Into Steel, Nuclear Instrum. Methods Phys. Rese. Sect. B Beam Interact. Mater. Atoms, 1985, 7, p 177–183.
B. Stypula and J. Stoch, The Characterization of Passive Films on Chromium Electrodes by XPS, Corros. Sci., 1994, 36(12), p 2159–2167.
B.R. Strohmeier, Characterization of an Activated Alumina Claus Catalyst by XPS, Surf. Sci. Spectra, 1994, 3(2), p 141–146.
D.B. Lee and S.W. Park, Oxidation of Cr2AlC Between 900 and 1200 °C in Air, Oxidat. Met., 2007, 68(5–6), p 211–222. https://doi.org/10.1007/s11085-007-9071-0
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for Publication
During the preparation of this work the author(s) used Quill bot] in order to improve the readability and grammar. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desai, V., Shrivastava, A., Zala, A.B. et al. Manufacturing of High Purity Cr2AlC MAX Phase Material and Its Characterization. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09513-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09513-4