Abstract
The aim of the work was to obtain a dense and uniform calcium phosphate (Ca-P) coating on the studied magnesium (Mg) alloy using simple methods that are easy to implement on an industrial scale. In this work, Ca-P layers were prepared on the surface of a Mg alloy. The simple wet chemical method based on immersion in an aqueous solution was used to prepare the Ca-P layer on the Mg alloy (AM60) surface. The effect of chemical modification by potassium chloride (KCl) and potassium nitrate (KNO3), as well as annealing on the morphology of the phosphate layers on the AM60 alloy, was determined, as well as the impact of this layer on the evolution of hydrogen in Ringer’s solution. The addition of KCl and KNO3 to the phosphating bath caused coagulation and agglomeration of the elements of the Ca-P coating. Consequently, the flake structure of the Ca-P coating changes into two types of structures: chrysanthemum and rhombohedral. Annealing at 150 °C for 3 h allows one to obtain a dense and uniform Ca-P coating on the studied Mg alloy. The Ca-P coating obtained by annealing at 150 °C can greatly decrease the hydrogen evolution rate of AM60 alloy in Ringer’s solution to 0.02 ml/cm2/day, which is similar to the safe amount of hydrogen for the human body (H2 ≈ 0.01 ml/cm2/day).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the field of medicine, particularly in implantology, there is a constant need to introduce improvements in simple technological solutions facilitating surgical techniques. Hence, the intensified work of scientists to broaden the spectrum of resorbable implants significantly simplifies the course of implantation, treatment, and rehabilitation of patients. Resorbable implants reduce the implantation step from two to one implant insertion. Moreover, it is not necessary to remove the corroded, non-resorbable implant in the second operation after bone fusion. Alternative non-inert biomaterials for short-term orthopedic implants include magnesium (Mg) alloys. Mg and its alloys have gained significant attention as medical implants for a broad range of clinical applications, including load-bearing applications, due to their biocompatibility, low density, and Young’s modulus, which is similar to cortical bone. A similar modulus of elasticity between bone and Mg alloys would minimize the phenomena of stiffing. Stiffing leads to a reduction in the density and weakness of bone (Ref 1).
However, there is a barrier to using biocompatible Mg alloys as a biomaterial for a short-term implant. Mg is a chemically active metal, and the degradation under physiological conditions (i.e., in vitro tests) is too fast. Attempts have been made to control the degradation process of Mg alloys by altering the chemical composition with rare earth metals (Ref 2), noble metals (Ref 3) addition, or by modifying the production method (Ref 4). However, these solutions are not fully effective because they do not provide a surface structure that guarantees acceptable corrosion progress or bioactive functions. In terms of the interaction of the implant with tissue, mainly bone, the surface of the implanted material plays a key role (Ref 5). Determining the best possible combinations of materials’ implant properties involves not only research on new alloys but also all of the modifications of their surface layer. Because the degradation process begins at the interface of the implant surface and body fluid or tissues, the most effective and simplest way to protect the implant against too rapid degradation of the core, whose main task is to transfer loads, is the biofunctionalization of the Mg alloy surface (Ref 6). The use of coatings that slow down the hydrogen release process and may possess bioactive properties, with a similar chemical composition to human bone, accelerates the process of bone union at the fracture site.
The combination of an Mg alloy substrate and a bioactive coating is a prospective research direction. Currently, various types of ceramic layers are used to increase corrosion resistance and delay the degradation of Mg alloy. The ceramic layers include oxides (Ref 7), carbides (Ref 8), nitrides (Ref 9), fluorides (Ref 10), and calcium phosphates (Ca-Ps) (Ref 11). Ca-P’s are commonly used for protective layers. They are present naturally in several biological structures, e.g., teeth and bones. Due to its bioactivity, biocompatibility, and osteoconductivity, Ca-P bioceramics are used as a substitute for tissues, wherever it is necessary to rebuild damaged bone. Additionally, Ca-P biodegradation occurs through the release of Ca and P, which further supports the healing process (Ref 12).
In addition, during the degradation of Mg and its alloys, hydrogen gas is released. The formation of hydrogen gas bubbles around the implant could delay tissue healing. The hydrogen evolution increases the pH of body fluids, which causes a local alkalization in the implant environment (Ref 13, 14). There is also the possibility that a significant amount of hydrogen gas will not be processed by the tissues, resulting in embolism and death of the patient. This is why it is so important to find a way to control the amount of hydrogen released. Therefore, the primary goal of this research is to determine the amount of hydrogen released over time. The use of coatings that will protect the body against the accumulation of hydrogen. Additionally, the biomimicry of the coatings, i.e., the similarity in chemical composition to human bone, will allow for control over the release of gaseous hydrogen and accelerated bone growth at the fracture site. In this work, the ideal hydrogen evolution rate was determined to be 0.01 (ml/cm2)/day because this amount of hydrogen can be transported away from the place of its production (Ref 15).
Because hydrogen can be harmful to the health and life of the patient as a degradation product of Mg alloy, this work focused on examining to what extent the newly developed Ca-P layer on the Mg alloy will be effective at protecting against too much hydrogen being released. Therefore, the evaluation criterion was the amount of hydrogen released by the sample with a Ca-P layer, which should be the closest to the 0.01 ml/cm2/day rate. The Mg alloy AM60 was used as the substrate for the tests. The AM60 alloy is a material that allows for a quick assessment of the effectiveness of Ca-P coatings because its degradation is accompanied by a significant amount of released hydrogen. In addition, Ringer’s solution was chosen as the dissolution media because its chemical composition does not contain phosphates or acetates, which could influence other dissolution mechanisms of the tested alloys (Ref 16, 17). Moreover, the presence of chlorides (representing an aggressive environment) in Ringer’s solution enables testing conditions that accelerate the dissolution mechanisms, which, in turn, enables the assessment of the weaknesses of the test material in a short time.
In this work, Ca-P layers were prepared on the surface of an Mg alloy using a simple chemical wet method. As a result, the technological process does not require complicated equipment and can be used directly in the medical industry. The chemical method based on immersion in an aqueous solution was used to prepare Ca-P layers on the Mg alloy (AM60) surface. The scientific aim of the work was to determine the effect of chemical modification by potassium chloride (KCl), potassium nitrate (KNO3), and annealing on the morphology and properties of phosphate layers on AM60 alloy. Potassium compounds such as KCl (used in medicine as a drip source of potassium for the patient) and KNO3 (used in the food industry as a food preservative) were used in the work. Potassium compounds were used in testing due to the potential use of the tested materials as implant materials for the treatment of bone fractures. Potassium is needed for the proper course of building bone tissue. Moreover, potassium reduces urinary calcium excretion (Ref 18).The criterion for the selection of compounds was the potential non-toxicity (KNO3) and probable biocompatibility (KCl) for the human body. The rest of the components (Table 1) for the solution were selected based on a literature review on the chemical composition of solutions for the preparation of phosphate layers. The research aim of the work was to obtain dense and uniform Ca-P coating on the studied Mg alloy by adjusting the chemical composition of the phosphating bath and parameters of annealing.
2 Materials and Methods
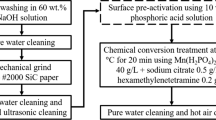
The AM60 die-cast Mg alloy (chemical composition: 4 wt.% Al, 0.3 wt.% Mn, 0.2 wt.% Zn, rest Mg) was used in experiments as the substrate, and the sample size was 35 mm × 25 mm × 5 mm. The sample surface was mechanically ground up to 600 grit, cleaned using distilled water and ethanol, and then dried in open air. The Ca-P layers were prepared using a wet chemical method. The samples were immersed in a beaker containing solution 1, 2, 3, or 4 (Table 1) for 24 h at room temperature to prepare the coating. In the second step, the coated Mg samples were dried and kept in the air for 24 h at room temperature. Chemical reagents with a high purity were used to prepare solutions 1-4.
For sample AII, annealing was performed after immersion in solution 2. After removing the samples from the solution, they were placed in a furnace heated to 150 °C. The samples were kept in the furnace at this temperature for 3 h. After this time, the samples were removed from the furnace and air-cooled. The obtained phosphate coatings structure and post-corrosion tests were conducted using scanning electron microscopy (SEM Supra 35 Carl Zeiss) after 120 h of immersion in Ringer’s solution to examine the corrosion products and chemical composition of the corroded surfaces. The thickness of the obtained coatings was measured on sample cross sections using Axiovision Rel. 4.4 software (Zeiss). A Panalytical X’Pert Pro MPD diffractometer with filtered radiation of a cobalt-anode lamp (λKα = 0.179 nm) and a PIXcell 3D detector on the diffracted beam axis was used to analyze the layer structure and corrosion products. Qualitative phase analysis of the samples was also performed based on x-ray diffraction measurements. Difractograms were recorded in the angular range of 10°-100° 2θ at a step of 0.05°. Analyses were performed using Panalytical High Score Plus software with a dedicated PAN-ICSD database. The card numbers: Mg—98-016-8829, Mg(OH)2—98-016-9979, MgO—98-015-7528, CaHPO4—98-000-0918, CaCO3—98-016-1820. The database the cards come from is ICSD. The phase analysis of the substrate and coated AM60 samples was conducted before and after immersion tests. The immersion tests and measurements of the released hydrogen volume of the studied samples were carried out in Ringer’s solution (8.6 g/dm3 NaCl, 0.3 g/dm3 KCl, 0.48 g/dm3 CaCl2·6H2O) for 120 h. To measure the volume of hydrogen evolution, a previously established experimental setup was used (Ref 19). The immersion tests were carried out at 37 °C.
3 Results
In Fig. 1, the surface morphology of AI, AII, AIII, and AIV samples was presented using SEM.
The layers obtained on samples AI and AII are very similar. They are characterized by a petal-like morphology. The petals are arranged in several directions (Fig. 1a, b, g, h). The arrangement of the layer elements on the AII sample may indicate the growth of the layer that began to nucleate on the AI sample (the beginnings of a similar morphology are indicated in the squares in Fig. 1a). Samples AIII (addition of KCl) and AIV (addition of KNO3) have a different morphology than the layers on the rest of samples. On the AIII sample surface, the chrysanthemum morphology is visible and likely composed of petal agglomeration. In turn, the layer elements on the AIV sample are much thinner and longer than in the layer formed on the AI sample.
Figure 2 presents light microscopy images of AI (a, b), AII (c, d), AIII (e, f), and AIV (g, h) sample cross sections. Based on light microscopy observations, it can be concluded that all the tested samples with Ca-P coatings are non-uniform in thickness. The thickness of the layers was measured in several places, but these results are varied and can only estimate the approximate thickness of the layers.
The x-ray phase analysis for AM60 (substrate) and the AI, AII, AIII, and AIV samples is presented in Fig. 3. The phase composition analysis of the AM60 substrate showed the presence of α-Mg phase mainly, which is consistent with the Mg-Al system. Diffraction lines characteristic of a calcium dihydrogen phosphate (CaHPO4) structure were identified on the diffractograms recorded for the AI, AII, AIII, and AIV samples. Diffraction lines derived from the substrate (α-Mg phase) were also identified. The results of the x-ray phase analysis of the AII sample also indicated the probable presence (only two small peaks) of calcium carbonate (CaCO3).
The hydrogen evolution rate in Ringer’s solution at 37 °C for AM60 alloy and the coated AI, AII, AIII, and AIV samples are presented in Fig. 4 and Table 2.
The AM60 substrate has the highest hydrogen evolution rate values. Among the coated samples, sample AII had the lowest rate (0.02-0.03 ml/cm2/day), which was also closest to the ideal (0.01 ml/cm2/day) hydrogen evolution rate.
Table 3 presents degradation rate calculated by hydrogen evolution rate. For all tested samples, the determined degradation rate shows an increasing tendency with increasing immersion time. Calculated degradation rate for AM60 increases the fastest, although after 120 hours of immersion a slight reduction in Vcorr is visible. Probably the layer of corrosion products with such a large amount of released hydrogen is so dense that it blocks the progress of further corrosion and hinders the release of hydrogen. For samples AI, AII, AIII, AIV, the calculated degradation rate increases with the immersion time, although it indicates lower values than in the case of the AM60 sample. In the case of samples AI and AIII, after 96 h and 120 h of mmersion, a slight stabilization of the degradation rate is visible. In the case of AII and AIV samples, there is no such stabilization of the degradation rate. Probably the rate and progress of the degradation rate depend on the morphology of the Ca-P layers produced.
The cummulated hydrogen evolution in Ringer’s solution at 37 °C for the AM60 and coated AI, AII, AIII, and AIV samples are presented in Fig. 5. After 120-h immersion of the AM60 substrate, the largest volume of hydrogen among all samples was evolved (9.2 ml/cm2). After 120-h immersion, AI, AII, AIII, and AIV samples evolved hydrogen at 4.3, 4.1, 5.7, and 5.4 ml/cm2, respectively, after 120 h of immersion.
After 120 h of immersion in Ringer’s solution, SEM images of the surface morphology for AI, AII, AIII, and AIV samples were taken (Fig. 6). The surface structure of AI and AIV samples after immersion in Ringer’s solution is very similar. The surfaces of the samples are covered with corrosion products, and their layers have visible small cracks. The surface structure of the AII sample is the most homogeneous in terms of structure among the tested samples. Corrosion products formed an almost consistent layer. The surface of the AIII sample was covered with corrosion products that were arranged chaotically and formed discontinuities in the layer.
In Fig. 7, the x-ray phase analysis results for the AM60 alloy, AI, AII, AIII, and AIV samples after immersion in Ringer solution at 37 °C were presented. The phase analysis of the substrate after immersion tests mainly showed the presence of α-Mg and Mg hydroxide (Mg(OH)2). There are also low-intensity peaks from MgO. For coated samples, CaHPO4 and α-Mg were the main phases. In addition, Mg(OH)2 and MgO peaks were present on the diffractograms of the AI, AII, and AIV samples.
4 Discussion
The modification of the Ca-P layers is a very important problem to be solved, which will determine the course and progress of the degradation of Mg alloys that can be used in orthopedic implantology. The morphology of the layer has a significant impact on the effectiveness of the protection of Mg alloys against corrosion. Hiromoto et al. produced hydroxyapatite (HA) layers on the AZ31 alloy and pure Mg and found that the protection provided by the HA coating is related to the inner layer, and the effectiveness does not significantly depend on the type of Mg substrate (Ref 20). The inner layer of AZ31 consisted of domed and densely packed deposits.
The main objective of this work was to create dense layers with the most homogeneous morphology to ensure uniform degradation progress over the entire alloy surface. Many chemical reagents and technological operations have been used in the literature on the subject to obtain the most uniform protective layer for Mg alloy. In many papers, an alkaline solution and thermal treatments are indicated as interventions ensuring even Ca-P layers on Mg alloys will be obtained. For example, Lei et al. used a 10 M potassium hydroxide (KOH) solution in the anodic electrodeposition process and annealed at 450 °C for 6 h in air. In this method, a dense and evenly thick layer of Mg oxide (MgO) was obtained. Thus, there are reasons to use potassium compounds and annealing as effective layer modifiers for better protection of the surface of Mg alloys against corrosion (Ref 21). In this work, a simple method was identified to modify Ca-P layers, which will be easy to implement on an industrial scale. It was found that potassium compounds and annealing can modify the morphology of Ca-P layers and improve corrosion properties, particularly by reducing the hydrogen evolution rate in Ringer’s solution of AM60 alloy. Annealing the Ca-P layer on the surface of AII samples resulted in the growth of the layer, which can be seen in the image of the AI sample surface (marked areas, Fig. 1a). As a result, the layer is more dense and uniform. The addition of KCl to the phosphatizing bath significantly changed the morphology of the layer formed on the AM60 alloy. One can assume that KCl caused the agglomeration of the petals into a chrysanthemum form. In turn, the addition of KNO3 (AIV sample) coagulated the phosphate layer flakes compared to the AI sample. Therefore, potassium compounds likely cause coagulation or agglomeration of the phosphate layer elements. The coagulation and agglomeration of flakes contributed to the irregularity of the layer thickness (Fig. 2e-h). The largest irregularity of thickness was identified for the AIII and AIV samples after estimating the layer thickness.
The results of x-ray examinations (Fig. 3) indicated that the layers on AI, AII, AIII, and AIV are mainly composed of dicalcium phosphate dihydrate (CaHPO4·2H2O, DCPD). In work (Ref 22), several reactions that occur during the deposition of a Ca-P layer on the surface of a Mg alloy were determined. These are the ionization of phosphoric acid molecules and the simultaneous dissolution of Mg ions in the phosphating solution, resulting in the formation of a large amount of hydrogen and OH ions. In the work (Ref 23), the authors explain that the potential of magnesium is extremely negative; therefore in the corrosive solution, the α-Mg phase acts as an anode, and the other phases act as a cathode in the electrochemical reaction. Hence, the α-Mg phase dissolves to form Mg2+ and hydrogen and OH− are formed at the cathode. In acidic calcium-phosphorus solutions at pH 1.8, a high concentration of Mg2+ first inhibits the binding of Ca2+ to ionize HPO42− and then the binding of Mg2+ to H2PO4− to produce MgHPO4, which acts as a precursor covering the magnesium alloy. Taking into account the free energy change equation in a supersaturated solution, it is easiest to generate the precipitation of Ca3(PO4)2 and DCPD. Therefore, Ca3(PO4)2 and DCPD crystals are formed in the CaP conversion solution and attached to the alloy surface.
DCPD is biologically important because it takes part in biomineralization processes such as bone formation (Ref 24). Therefore, it is possible that the DCPD layers obtained in this work will be able to perform a bioactive function and support or even accelerate the process of bone formation at the fracture site.
For comparison, the flake-like structure of the DCPD layer on Mg alloy was produced by Y. Song using the electrodeposition method (Ref 25). In another study, ZK60 alloy produced a Ca-P layer by chemical conversion (Ref 26). In this work, x-ray analysis showed that the layers formed on the Mg alloy consisted of DCPD. Furthermore, as a result of heat treatment, DCPD transformed into HA (Ref 26). In addition, this process was accompanied by the formation of the Ca2P2O7 phase. In this work, no HA layer was formed on the annealed sample. The different annealing temperatures in the current study (150 °C) and Wang et al. (350 °C) caused a lack of transformation of DCPD to HA in this work. The selected 150 °C annealing temperature was chosen because the priority was to maintain the substrate input structure (i.e., no phase changes). The annealing temperature was chosen based on the Mg-Al phase system. Based on the Mg-Al phase system, there should be no change in the phase composition of the AM50 alloy by annealing up to ~ 200 °C. No change in the phase composition of the substrate after annealing of the AII sample was confirmed (Fig. 3). No new phases originating from the substrate were observed after comparing the diffraction patterns of the substrate and the substrate with the AII layer. The purpose of the substrate coating treatments was to maintain the microstructure and phase composition of the AM50 alloy. The study of Ca-P coatings, which show chemical similarity to bone minerals, is justified and important due to the probable bioactivity that shortens the time of bone union. However, Zaludin et al. produced coatings of DCPD on an Mg substrate and concluded that the low porosity of this coating helped minimize the effects of penetration ions from the solution (Ref 27).
Because the risk of hydrogen evolution on human health directly depends on the temporal and spatial rate of its release, the hydrogen evolution rate on every day of immersion was determined (Fig. 4; Table 2). The local buildup of a large volume of gas will not occur if the release rate is 0.01 (ml/cm2)/day (Ref 15). The results of the hydrogen evolution rate for the AII sample were closest to this value. Unfortunately, the determined values of the hydrogen evolution rate for the AII sample (Table 2) have an increasing trend, although stable. The longer the sample was immersed in Ringer’s solution, the higher the rate of hydrogen evolution. Hydrogen evolution is a cathodic reaction during the corrosion of Mg-based alloys. Therefore, the amount of hydrogen evolution can be used to determine the corrosion resistance of these materials (Ref 28, 29). It should be noted that all the DCPD layers obtained in this work caused a decrease in the amount of evolved hydrogen during immersion in Ringer’s solution. The reference sample, uncoated AM60 alloy, was corroded rapidly, producing a large amount of hydrogen (Fig. 5). DCPD layers are effective in reducing hydrogen evolution. In (Ref 28) and (Ref 29), DCPD layers were produced on AZ91D alloy and pure Mg, which reduced the amount of hydrogen evolution during immersion in simulated body fluid several times. Therefore, the layers composed of DCPD constitute an effective barrier against the progress of corrosion in artificial body fluid environments. HA is the most desirable form of Ca-P due to its excellent biocompatibility, bioaffinity, and bone conductivity. However, the synthesis of HA by precipitation leads to the formation of agglomerate crystals, the size of which is often difficult to control. By using DCPD as a precursor, it is possible to control the crystal size of DCPD and then convert it directly to HA (Ref 30). DCPD is often used as an initial ingredient in bone cements and is a promising coating for clinical applications. DCPD is also biocompatible with various cell lines, such as mouse fibroblasts.
In this work, the hydrogen evolution course for the AM60 alloy and AI and AII samples was linear. In the case of AIII and AIV samples, the hydrogen evolution course had a more interval character. Periods of very intense hydrogen evolution and periods of slight hydrogen release were observed (Fig. 5). Since Mg reacts with water to release not only Mg ions but also hydrogen gas that may disturb the Ca-P coating, thus destroying the compactness and homogeneity of as-deposited coatings (Ref 31). Consequently, the irregularity of the layer thickness in the AII and AIV samples resulted in the formation of areas that are easier for the chlorides present in the Ringer’s solution to penetrate. As a result, the linear corrosion progress and continuous layer formation of corrosion products on the entire surface of the samples are not possible. This is confirmed by the results of surface tests after 120 h of immersion in Ringer’s solution (Fig. 6). On the surface of the AI and AII samples, the corrosion products form a consistent layer (Fig. 6a-d). Therefore, the degradation process of the Ca-P layer and simultaneously the formation of the corrosion products layer is gradual. In turn, discontinuities and random distribution of corrosion products are visible on the surface of the AIII and AIV samples (Fig. 6e-h). The XRD results after 120 h of immersion in Ringer’s solution indicated the presence of DCPD in the surfaces of samples with Ca-P layers (Fig. 6). On the surface of the AM60 uncoated sample, the presence of mainly Mg and Mg(OH)2 phases was confirmed. The peaks of Mg(OH)2 indicated the formation of an alkalized layer. Two peaks of Mg(OH)2 also appeared on the diffraction patterns of coated samples. These peaks are likely associated with some small areas of an alkalized layer on the surface-coated samples. In sum, the layer composed of DCPD had better protection properties against the progress of the corrosion process than Mg(OH)2. Both annealing and the use of potassium compounds affected the morphology of the DCPD layer and increased corrosion resistance. In terms of remodeling the morphology of the DCPD layer, potassium compounds are more effective. In terms of increasing the corrosion resistance of the Mg alloy-DCPD layer system, adjusting annealing conditions (150 °C for 3 h) is more favorable in relation to the tested materials. With regard to the adhesion of these Ca-P layers, they must have high solubility in aqueous solutions. Given the environment of body fluids, the adhesion of the coating will decrease over time after implantation. This would be a favorable situation due to the biocompatible chemical composition of the Ca-P layers with bone tissue. In such a case, the adhesion decreasing over time would activate the processes of promoting the growth of new bone tissue at the fracture site.
5 Conclusions
The DCPD layers performed in this work reduced the amount and rate of hydrogen evolution during immersion in Ringer’s solution. The main conclusions are:
-
Adding potassium compounds, such as KCl and KNO3, to the phosphatizing solution led to more agglomerated and coagulated Ca-P layers.
-
Annealing the Ca-P layer on the surface of the AII sample resulted in the growth of the layer.
-
The most effective way to reduce the amount of hydrogen produced is annealing (150 °C for 3 h) in relation to the tested materials.
-
The layers prepared on the studied samples were mainly composed of DCPD.
-
DCPD is biologically important because it takes part in biomineralization processes such as bone formation. Therefore, it is possible that the DCPD layers obtained in this work will be able to perform a bioactive function and support or even accelerate the process of bone formation at the fracture site.
References
V. dos Santos, R.N. Brandalise, and M. Savaris, Engineering of Biomaterials Springer, 2017
A. Kania, R. Nowosielski, A. Gawlas-Mucha, and R. Babilas, Mechanical and Corrosion Properties of Mg-Based Alloys with Gd Addition, Mater., 2019, 12(11), p 1–16.
D. Szyba, D. Bajorek, D. Babilas, L. Temleitner, D. Łukowiec, and R. Babilas, New Resorbable Ca-Mg-Zn-Yb-B-Au Alloys: Structural and Corrosion Resistance Characterization, Mater. Des., 2022, 213, p 1–16.
S. Lesz, B. Hrapkowicz, M. Karolus, and K. Gołombek, Characteristics of the Mg-Zn-Ca-Gd Alloy After Mechanical Alloying, Materials, 2021, 14(1), p 1–14.
A. Liber-Kneć and S. Łagan, Właściwości powierzchni tkanek i implantów, in: A. Liber-Kneć, S. Łagan, editors. Metody badań biomateriałów I tkanek. Wstęp do ćwiczeń laboratoryjnych. Crocov University of Technology, Kraków, 2020. pp. 85–93
Y. Su, I. Cockerill, Y. Zheng, L. Tang, Y. Qin, and D. Zhu, Biofunctionalization of Metallic Implants by Calcium Phosphate Coatings, Bioact. Mater., 2019, 4, p 196–206.
A. Kania, P. Nolbrzak, A. Radoń, A. Niemiec-Cyganek, and R. Babilas, Effect of the Thickness of TiO2 Films on the Structure and Corrosion Behavior of Mg-Based Alloys, Mater., 2020, 13(5), p 1–14.
L. Yang, Z. Li, Y. Zhang, S. Wei, and F. Liu, Al-TiC In Situ Composite Coating Fabricated by Low Power Pulsed Laser Cladding on AZ91D Magnesium Alloy, Appl. Surf. Sci., 2018, 435, p 1187–1198.
D.V. Mashtalyara, S.L. Sinebryukhova, I. Imshinetskiya, A. Gnedenkova, and K. Nadaraiaa, Hard Wearproof PEO-Coatings Formed on Mg Alloy Using TiN Nanoparticles, Appl. Surf. Sci., 2020, 503, p 1–12.
D.B. Panemangalore, R. Shabadi, M. Gupta, and G. Ji, Effect of Fluoride Coatings on the Corrosion Behavior of Mg–Zn–Er Alloys, Surf. Interfaces, 2019, 14, p 72–81.
L. Wang, X. Xiao, E. Liu, S. Yu, X. Yin, J. Wang, G. Zhu, Q. Li, and J. Li, Fabrication of Superhydrophobic Needle-Like Ca-P Coating with Anti-fouling and Anti-corrosion Properties on AZ31 Magnesium Alloy, Colloids Surf. A Physicochem. Eng. Aspects, 2021, 620, p 1–11.
N. Eliaz and N. Metoki, Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coat. Technol. Biomed. Appl. Mater., 2017, 10(4), p 1–104.
A. Atrens, S. Johnston, Z. Shi, and M.S. Dargusch, Viewpoint—Understanding Mg Corrosion in the Body for Biodegradable Medical Implants, Scr. Mater., 2018, 154, p 92–100.
V. Kumar, B. Mallarap, G. Krishna, and R. Tirumala, Magnesium Matrix Composites for Biomedical Applications: A Review, J. Magnes. Alloys, 2019, 7(1), p 72–79.
S. Amukarimi and M. Mozafari, Biodegradable Magnesium-Based Biomaterials: An Overview of Challenges and Opportunities, MedComm, 2021, 2, p 123–144.
D. Cao, L. Wu, Y. Sun, G. Wang, and Y. Lu, Electrochemical Behavior og Mg-Li, Mg-Li-Al and Mg-Li-Al-Ce in Sodium Chloride Solution, J. Power. Sources, 2008, 177, p 624–630.
G. Song and A. Song, A Possible Biodegradable Magnesium Implant Material, Adv. Eng. Mater., 2007, 9, p 221–327.
M.S. Stone, L. Martyn, and C.M. Weaver, Potassium Intake, Bioavailability, Hypertension, and Glucose Control, Nutrients, 2016, 8(7), p 1–13.
K. Cesarz-Andraczke, R. Nowosielski, M. Basiaga, and R. Babilas, Study of the Morphology and Properties of Biocompatible Ca-P Coatings on Mg Alloy, Materials, 2020, 13(2), p 1–13.
S. Hiromoto and M. Tomozawa, Hydroxyapatite Coating of AZ31 Magnesium Alloy by a Solution Treatment and Its Corrosion Behavior in NaCl Solution, Surf. Coat. Technol., 2011, 205, p 4711–4719.
T. Lei, H. Ouyang, W. Tang, L.F. Li, and L.S. Zhou, Enhanced Corrosion Protection of MgO Coatings on Magnesium Alloy Deposited by an Anodic Electrodeposition Process, Corros. Sci., 2010, 52, p 3504–3508.
X. Liu, X. Wang, L. Ren, Y. Dai, J. She, F. Qi, W. Wei, D. Zhang, and X. Ouyang, Improved Deposition Quality of Calcium-Phosphate Coating on the Surface of WE43 Magnesium Alloy via FCVA Sputtering Pretreatment, J. Market. Res., 2023, 26, p 6672–6688.
X. Wang, X. Liu, Y. Dai, J. She, D. Zhang, F. Qi, W. Wei, and X. Ouyang, A Novel Ca-Mg-P/PDA Composite Coating of Mg Alloys to Improve Corrosion Resistance for Orthopedic Implant Materials, Surf. Coat. Technol., 2023, 47, 129920.
B.Q. Lu, T. Willhammar, B.B. Sun, N. Hedin, J.D. Gale, and D. Gebauer, Introducing the Crystalline Phase of Dicalcium Phosphate Monohydrate, Nat. Commun., 2020, 11, p 1–8.
Y. Song, S. Zhang, J. Li, C. Zhao, and X. Zhang, Electrodeposition of Ca–P Coatings on Biodegradable Mg Alloy: In Vitro Biomineralization Behavior, Acta Biomater., 2010, 6, p 1736–1742.
B. Wang, P. Huang, C. Ou, K. Li, B. Yan, and W. Lu, In Vitro Corrosion and Cytocompatibility of ZK60 Magnesium Alloy Coated with Hydroxyapatite by a Simple Chemical Conversion Process for Orthopedic Applications, Int. J. Mol. Sci., 2013, 14, p 23614–23628.
M.A. Zaludin, Z.A. Jamala, M.N. Derman, and M.Z. Kasmuin, Fabrication of Calcium Phosphate Coating on Pure Magnesium Substrate via Simple Chemical Conversion Coating: Surface Properties and Corrosion Performance Evaluations, J. Mater. Res. Technol., 2019, 8(1), p 981–987.
S. Brundavanam, G.E. Poinern, and D. Fawcett, Chemical Immersion Coatings to Improve Biological Degradability of Magnesium Substrates for Potential Orthopaedic Applications, J. Biomed. Mater. Res., 2014, 2(2), p 7–14.
Z.J. Cheng, H.Y. Lian, and G. Li, Biocompatible DCPD Coating Formed on AZ91D Magnesium Alloy by Chemical Deposition and Its Corrosion Behaviors in SBF, J. Bionic Eng., 2014, 11, p 610–619.
H.R. Bakhsheshi-Rad, E. Hamzah, S.N. Saud, and M. Medraj, Effect of Electrodeposition Parameters on the Microstructure and Corrosion Behavior of DCPD Coatings on Biodegradable Mg–Ca–Zn Alloy, Int. J. Appl. Ceram. Technol., 2015, 12, p 1054–1064.
S. Jiang, S. Cai, Y. Lin, X. Bao, R. Ling, D. Xie, J. Sun, J. Wei, and G. Xu, Effect of Alkali/Acid Pretreatment on the Topography and Corrosion Resistance of as-Deposited CaP Coating on Magnesium Alloys, J. Alloys Compd., 2019, 793, p 202–211.
Acknowledgments
The publication was financed by the statutory grant of the Department of Engineering Materials and Biomaterials of the Faculty of Mechanical Technology at the Silesian University of Technology in 2023.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cesarz-Andraczke, K., Paczuła, D. & Nuckowski, P.M. KCl, KNO3, and Annealing for Modifying the Morphology and Properties of Ca-P Layers on Mg Alloy. J. of Materi Eng and Perform 32, 11171–11180 (2023). https://doi.org/10.1007/s11665-023-08903-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08903-4