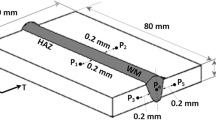
Abstract
This research aimed to investigate the heat-affected zone of lean duplex stainless steel grade X2CrNiN22-2. Different heat-affected zone microstructures and grain morphologies were developed by Gleeble simulations. The governing microstructures were evaluated by metallographic techniques and electrochemical corrosion measurements. It was found that the 1200-800 °C cooling time significantly affects the microstructure, austenite content, and corrosion properties. The average austenite content in the case of 1 s cooling time is 30.7 ± 1%, which increased with the longer cooling times up to 38.6 ± 0.9%. The rapid cooling times resulted in a more ferritic microstructure, which promoted nitride precipitation in the ferrite grains. The nitride precipitations acted as nucleation sites for pitting initiation in 3.5 wt.% NaCl solution. The lowest pitting potential was measured in the case of the most rapidly cooled sample: 573 ± 31 mV, while the balanced, annealed microstructure had much better pitting corrosion resistance, showing a pitting potential of 1308 ± 62 mV vs. the Ag/AgCl (KCl sat.) reference electrode. The results of this research can be used in designing welding parameters for the welding of the X2CrNiN22-2 lean duplex stainless steel.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Duplex stainless steels (DSS) have higher strength (Ref 1, 2) and higher corrosion resistance to pitting and stress corrosion cracking than conventional austenitic or ferritic stainless steels (Ref 3,4,5,6,7,8). Recently, new generations of DSS called lean duplex stainless steels (LDSS) have been developed with lower nickel (Ni) and molybdenum (Mo) content (Ref 9, 10). These types of grades are usually used as a substitution for conventional austenitic grades, such as 304 L, with the same pitting corrosion resistance but with double yield strength (Ref 11). The higher strength makes them applicable and sustainable to construct storage tanks, pipelines, and pressure vessels with thinner wall thickness (Ref 12). Also, the lower Ni and Mo content makes them a cost-effective solution for chemical (Ref 13), oil and gas (Ref 14), and civil engineering industries, and however, their application is still around 1% among stainless steels (Ref 15,16,17).
One of the reasons for this is the weldability issues. The thermal impact of a welding process can significantly differ in the austenite/ferrite (A/F) phase balance in the weld metal (WM) and heat-affected zone (HAZ) (Ref 18,19,20). DSSs are also sensitive to intermetallic formation due to slow cooling rates or multiple thermal cycles. This change in the phase ratio and the microstructure affects the mechanical and corrosion properties (Ref 21, 22), leading most DSS failures to occur in the HAZ or WM (Ref 23).
Electrochemical corrosion tests are a popular technique among researchers and steel manufacturers to characterize the local corrosion resistance of DSS. Alvarez et al. (Ref 24) showed that corrosion resistance depends on the ferrite-to-austenite ratio and the chemical composition of the ferrite and austenite phases. Their research showed that lean duplex grade 2304 preferentially corrodes in the ferrite phase, while in the newly developed 2001 lean duplex grade, the austenite phase showed lower resistance in alkaline solutions. Zhang et al. (Ref 25) performed detailed investigations on the localized corrosion resistance of lean duplex grade 2101. They found that pitting corrosion was initiated preferentially at the ferrite–austenite grain boundaries or inside the ferrite grains. Örnek et al. (Ref 26) performed atmospheric corrosion tests in a chloride-containing environment at 50 °C on 2202 (X2CrNiN22-2) and 2205 duplex grades. They found that the lean duplex grade 2202 shows lower corrosion resistance than standard duplex grade 2205. Guilherme et al. (Ref 27, 28) have found that the most critical part of a weld regarding corrosion resistance is the fusion line because of a Cr and Mo depleted zone. Thus, the fusion line and the high-temperature heat-affected zone (HTHAZ) must be characterized to design welding procedures.
The ferrite-to-austenite solid-state phase transformation in the HAZ is mainly driven by nitrogen (N) diffusion (Ref 29). Thus, this phase transformation significantly depends on the HAZ's cooling rate. The lower cooling rates will result in higher austenite and lower ferrite content (Ref 30, 31). Low heat input welding processes like laser and electron beam welding will produce a highly ferritic HAZ and WM (Ref 32,33,34). To quantify the cooling rate and time in the HAZ the 1200 to 800 °C (Δt12/8) cooling time is used (Ref 35).
To investigate the HAZ of duplex stainless steels, Gleeble physical simulations are widely used among researchers and steel manufacturers (Ref 35,36,37). The physically simulated HAZs can be tested with different metallographic techniques and electrochemical corrosion measurements, contrary to the arc weld HAZs, which are usually a couple of hundred micrometers narrow (Ref 38).
In this paper, different HAZ morphologies and microstructures of lean duplex grade X2CrNiN22-2 were investigated by metallography and corrosion testing, and conclusions were drawn regarding HAZ properties.
2 Materials and Methods
2.1 Duplex Stainless Steel Base Material
The chemical composition of X2CrNiN22-2 (type 2202), 3.0 mm thick lean (low Ni and Mo content) duplex stainless steel sheet used for HAZ physical simulation can be seen in Table 1. The proof strength of the base material in the annealed state is Rp0.2 = 450 MPa, the tensile strength is Rm = 650 MPa, and the elongation is A = 30%. The chemical composition was measured by PMI Master Sort and SPECTROTEST optical emission spectroscopes.
The equilibrium microstructure of X2CrNiN22-2 grade DSS during cooling was calculated by OpenCalphad V6.0 thermodynamic software can be seen in Fig. 1. After solidification, the microstructure remains fully ferritic down to 1330 °C. The ferrite-to-austenite phase transformation mainly taking place in the 1200-800 °C temperature range. At the high temperature range of the HAZ M2(C, N) type carbides and nitrides (like Cr2N) can precipitate. Upon cooling, Cr-rich intermetallic phases like σ and α’ can form. At lower temperatures, π-nitrides can precipitate in the equilibrium state.
2.2 Gleeble Heat-Affected Zone Physical Simulations
To produce different HAZ microstructure Gleeble 3500 physical simulator was used. The 50 × 20 × 3 mm X2CrNiN22-2 DSS grade sheet samples were heated to 1350 °C in pure argon atmosphere to achieve a completely ferritic microstructure (as shown in Fig. 1). The 1350 °C peak temperature is found to be sufficient as a ferritization temperature for 22% Cr (Ref 35), 22% Cr and Mo-free (Ref 39), and 2% Ni lean duplex grades (Ref 37). The peak temperature was achieved in under 10 s. After 1 s holding time at the maximum temperature, 9 different Δt12/8 cooling times were utilized by different cooling methods (air cooling and forced water cooling). The Δt12/8 cooling times were 1, 3, 6, 8, 10, 12, 20, 30, and 50 s to investigate a wide range of cooling times and the governing microstructures (Fig. 2). There are different recommendations of heat input (or arc energy) ranges for the arc welding of DSS (Ref 40). Still, if the widest possible heat input range is considered (0.2-2.5 kJ/mm), then the cooling times in industrial welding applications are in-between approximately 1-12 s, considering a three-dimensional heat conductivity. From each set of experiments, 3 samples were produced. The setup can be seen in Fig. 3.
2.3 Metallographic Examinations of the Heat-Affected Zone Microstructures
The metallographic samples were cut out at the location of the thermocouples, perpendicularly to the longer side of the sheets. The cross sections were cold-mounted into epoxy resin and ground up to 4000 grit sandpaper under constant cooling. After grinding, the samples were polished with 3 µm and 1 µm diamond suspension. To determine the A/F ratio, color etching was used with Beraha’s reagent (Ref 41), consisting of 85 mL H2O + 15 mL HCl + 1 g K2S2O5 for 16 s. For A/F ratio measurement, image analysis was used based on the ratio between the light and dark areas histogram. The method of A/F ratio measurement with image analysis can be found in our previous paper (Ref 42).
To reveal the nitride precipitations, 10% concentrated oxalic acid electrochemical etching was used on the mounted and polished samples, with 5 V potential for 30 s (Ref 43). The oxalic acid electrochemical etching reveals the nitride precipitations only and shows a poor contrast between the ferrite and austenite phases. Scanning electron microscopy (SEM) was used on the etched specimens using a Zeiss EVO MA 10 type SEM for a more detailed examination of the nitrides. For the metallographic investigations, three samples were mounted from each cooling rate.
2.4 Electrochemical Corrosion Tests
Potentiodynamic corrosion tests were used according to ASTM G61 standard using a Biologic SP-150 potentiostat to investigate the corrosion resistance of different simulated HAZ microstructures. The electrolyte was 3.5% NaCl with an initial pH value of 6.0. The temperature during the tests was kept at 25 ± 2 °C. The electrolyte was de-aerated with pure argon purging for 1 h before the test. The argon purging was also maintained during the test. In the standard 3 electrode cell, the working electrode was the mounted sample consisting of the simulated HAZ or the annealed base material, the reference electrode was saturated silver chloride (Ag/AgCl (KCl sat.)), and the counter electrode was a platinum (Pt) mesh. Before mounting, the metal samples were covered in polyacrylate varnish to prevent crevice corrosion. The samples were polished with 3 µm diamond suspension prior to the tests. After the polishing, the samples were cleaned with acetone in an ultrasonic cleaner. The samples were tested right after cleaning. The resting time before the potential scanning was 1 hour to stabilize the open circuit potential. The initial potential for the polarization was selected as − 0.3 V from the open circuit potential to investigate the Tafel behavior of the working electrodes. The scanning rate was 1 mV s− 1, and the potential scanning lasted until 10 mA cm− 2 corrosion current was reached. The corrosion potential (Ecorr), corrosion current density (icorr), and pitting potential (Epit) were determined graphically from the Tafel curves, where Epit is the potential in which the corrosion current is sharply monotonic increasing, showing stable pit formation. The electrochemical corrosion tests were repeated five times for each sample.
3 Results and Discussion
3.1 Microstructures of Physically Simulated Heat-Affected Zones
The X2CrNiN22-2 type lean duplex stainless steel solidifies as delta ferrite (δ) and remains fully ferritic down to 1330 °C (in equilibrium condition), as shown in Fig. 1. Below 1330 °C, the ferrite-to-austenite (δ ➔ γ) phase transformation starts, resulting in the duplex microstructure. The δ ➔ γ solid phase transformation is mainly driven by the diffusion of the nitrogen atoms. The nitrogen diffusion coefficient is significantly different in δ and γ phases. Zhang et al. (Ref 44) specified different nitrogen diffusion coefficients for δ (Dδ) and γ (Dγ) phases in the case of duplex stainless steels:
The diffusion distance (d) can be calculated as:
Considering one-dimensional diffusion at 1330 °C temperature, the diffusion distances (dδ and dγ) can be calculated as a function of time (Fig. 4).
At the beginning of the δ ➔ γ phase transformation temperature, the nitrogen is significantly more diffusible in δ than in γ. As N is a potent austenite former element, the resulting ferrite-to-austenite ratio will depend on the cooling time. Consequently, shorter cooling times will result in more ferritic microstructure. On the other hand, longer cooling times will result in more austenitic microstructure, as the nitrogen atoms have more time to diffuse to the grain boundary austenite. The simulated HAZ microstructures after different Δt12/8 cooling times from 1350 °C peak temperature (fully ferritic microstructure) can be seen in Fig. 5.
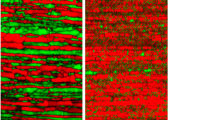
The darker areas are the ferrite, and the lighter areas are the austenite phases on the etched specimens. The as-received microstructure shows textured, elongated grains because of cold rolling. In the physically simulated HAZ microstructures, the shorter cooling times show more ferritic, and the longer cooling times show more austenitic microstructures. Also, different forms of austenite grain morphologies dominate in the different samples. In the more rapidly cooled samples, such as Δt12/8 = 1, 3 and 6 s, thin plates of grain boundary austenite (GBA) can be seen on the ferrite grain boundaries. As austenite former elements, such as N, Ni, and Mn, tend to segregate at the ferrite grain boundary, this is the first nucleation site of the solid-state δ ➔ γ phase transformation. The nitrogen trapped inside the ferrite grains will transform into smaller intergranular austenite (IGA) islands. With longer cooling times, Widmanstätten-type austenite (WA) tends to form from the GBA toward the center of the ferrite grains, such as in the case of the Δt12/8 12 s sample. With longer cooling times, the N atoms have more time to diffuse, thus significantly increasing the GBA width. Also, IGA formed inside the ferrite grains. The grain boundary austenite plate widths (GBAWs) and the ferritic area width in the prior fully ferritic grains (FGS) can be seen in Fig. 6 as a function of Δt12/8 cooling time.
As the cooling time increases, the austenite-promoting atoms (mostly the small nitrogen atoms) have more time to diffuse. The diffusion distance in ferrite can be significant. The N atoms diffuse to the formerly formed austenite grains and occupy the interstitial spaces in the austenite’s face-centered cubic crystal structure. As a result, the increasing cooling time means increasing GBA plate width (GBAW), as shown in Fig. 6. The error bars show the standard deviation of 40 measurements of GBAW and the ferritic area in the prior fully ferritic grains (FGS). In the sample with Δt12/8 = 1 s cooling time, the average GBAW is 16 ± 1 µm, which increases to 26 ± 3 µm after 12 s, and 37 ± 6 µm after 50 s cooling time. The grain boundary austenite width growth showing an exponential growth behavior with the increasing cooling time. The ferritic area size shows an opposite tendency. With the increasing cooling time, the ferritic area size slightly decreases as the grain boundary transforms into austenitic microstructure. The average ferritic area size with the Δt12/8 = 1 s cooling time is 130 ± 5 µm, which decreases to 119 ± 11 µm after 12 s, and 105 ± 8 µm after 50 s cooling time. The ferritic area size shows a slight exponential decay behavior with the increasing cooling time.
3.2 Nitride Precipitations in Physically Simulated Heat-Affected Zones
The high nitrogen alloying content can also have a detrimental effect if the microstructure is significantly ferritic. Otherwise, if the nitrogen atoms are dissolved in the austenitic phase, it significantly affects the pitting corrosion resistance and strength of DSS (Ref 45). The nitrogen solubility is substantially less in ferrite than in austenite. According to Lippold (Ref 46), the nitrogen solubility at 1200 °C is ~ 0.1 wt.% in ferrite and ~ 0.25 wt.% in austenite, which decreases to practically zero (less than 0.01 wt.%) solubility in ferrite below 700 °C, while austenite can dissolve 0.1 wt.% even at 500 °C. If the nitrogen atoms cannot occupy interstitial spaces in the austenite’s crystal structure and get trapped in ferrite, nitrides (mostly Cr2N, and CrN chromium-nitrides) will precipitate. Chromium nitrides can be shown by electrochemical etching techniques, listed in ISO 17781 standard. To reveal nitride precipitation in the ferrite grains, electrochemical etching was used with oxalic acid on the samples with Δt12/8 cooling times of 1, 12 and 50 s (Fig. 7). Oxalic acid attacks chromium-depleted regions surrounding the chromium nitrides.
As shorter cooling times resulting in more ferritic microstructure (as it can be seen in Fig. 5) the nitrogen solubility decreases. Nitrogen atoms tend to precipitate mostly as chromium-nitrides inside the ferrite grains. From Fig. 7, it can be seen that the most rapidly cooled sample (Δt12/8 = 1 s) shows larger ferrite grains (Fig. 6) and severe nitride precipitation (black spots on the etched images). As the austenite grains widen with the longer cooling times, the nitrogen needs to diffuse only shorter distances to occupy interstitial spaces and thus promote austenite formation. Consequently, less nitride precipitation is visible in Fig. 7 after the 12 s and 50 s cooling times. Figure 8 shows the secondary electron images of the most rapidly cooled (1 s) sample after the oxalic acid electrochemical etching.
In Fig. 8, the nitride precipitation sites are visible inside the ferrite grains and at the austenite–ferrite grain boundary. The nitrides precipitate in a dispersed distribution with tens of micrometers in size. As the chromium and nitrogen content (which are significantly responsible for the pitting corrosion resistance) is lower next to the precipitates (due to segregation), the nitrides act as a nucleation site for pitting corrosion mechanisms (Ref 47).
3.3 Austenite Content in the Physically Simulated Heat-Affected Zones
The result of the austenite content (A%) measurement in the physically simulated samples by image analysis can be seen in Fig. 9.
As the X2CrNiN22-2 lean duplex stainless steel is highly alloyed with nitrogen (0.22%), the austenite content does not get lower than 30%, even at rapid cooling rates, for the investigated 3 mm sheet thicknesses. The average austenite content in the case of Δt12/8 = 1 s is 31 ± 1%, which sharply increases with the longer cooling times up to 20 s, with austenite contents 32 ± 2% after 6 s, 36 ± 2% after 12 s, and 37.1 ± 0.8% after 20 s. The longer cooling times do not result in significant austenite formation. After 50 s cooling time, the average austenite content is 38.6 ± 0.9%. Westin et al. (Ref 48) found similar results of austenite content after the physical simulation of 1.52% Ni containing lean duplex stainless steel. The austenite increase in the X2CrNiN22-2 HAZ samples shows an exponential tendency, described with the following equation:
Regarding weldability, the HAZ of the grade X2CrNiN22-2 shows excellent behavior, as even at shorter cooling times (lower heat inputs), the austenite content remains above 30% on average.
3.4 Results of the Electrochemical Corrosion Tests
The potentiodynamic polarization curves of HAZ samples with 1, 12 and 50 s Δt12/8 cooling times and of the as-received (AR) base material can be seen in Fig. 10, along with the results listed in Table 2.
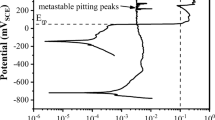
The heat treatment state and the governing microstructure significantly affect the corrosion resistance properties of lean duplex grade X2CrNiN22-2. The polarization curves were repetitively registered on each specimen, and the good reproducibility was confirmed. At the intersection of the cathodic (reduction) and anodic (oxidation) curves, the corrosion potential (Ecorr) can be defined. The Ecorr values varied in the range of − 65 ± 12 mV to − 241 mV ± 24 vs. the Ag/AgCl (KCl sat.) reference electrode in 3.5 wt.% NaCl solution at 25 °C. Small spikes in the oxidation region show metastable pitting formation in all specimens. The best (most positive) passive behavior was measured in the as-received state (AR), where the balanced austenitic–ferritic microstructure shows the best corrosion resistance. The corrosion potential is decreasing in the simulated HAZs. In the case of the most rapidly cooled sample (Δt12/8 = 1 s), the average Ecorr is − 181 ± 12 mV (austenite content A% = 30.7%), with 12 s cooling time is − 210 ± 14 mV (A% = 36.2%), and with 50 s cooling time is − 241 mV ± 24. Another important information that can be concluded from the polarization curves is the pitting potentials (Epit). A sharp increase in the corrosion current can be measured after the metastable pitting formation section in the anodic curves, where stable pitting formation starts (Fig. 11).
The higher Epit values conclude higher resistance against pitting corrosion. The balanced microstructure in the as-received (AR) state shows the best resistance against pitting corrosion in the 3.5 wt.% NaCl electrolyte. The average pitting potential (where a sharp increase is experienced in the corrosion current, i) was Epit = 1308 ± 62 mV vs. Ag/AgCl (KCl sat.) in the AR state, which is close to the results of Peultier et al. (Ref 11) in 30 g/L NaCl electrolyte. The simulated HAZ microstructures show reduced Epit values, meaning lower resistance against pitting corrosion. The average Epit values for Δt12/8 = 1 s are 573 ± 31 mV, for 12 s 706 ± 41 mV, and for 50 s 670 ± 33 mV. The lowest pitting potential was measured in the case of the most rapidly cooled sample, where the nitride precipitation shown in Fig. 7 and 8 acts as a nucleation site. The 12 s and 50 s samples show somewhat the same pitting potential, with an average difference of 36 mV. To examine the pitting formation with light optical microscopy (LOM) 10 mA·cm− 2 corrosion current density was selected as a threshold during the polarization tests. The microstructure after the corrosion tests can be seen in Fig. 12.
In the as-received state, the pits initiate in the ferrite grains with an average size of 60 ± 15 µm. With the balanced microstructure of elongated ferrite and austenite grains, the pits are initiated in the ferritic microstructure, where the nitrogen content is lower. Nitrogen is a potent alloying element that increases pitting corrosion resistance (Ref 13). A different pitting morphology can be observed in the most rapidly cooled sample (Δt12/8 = 1 s). In this case, the metastable and stable pitting formation initiated in scattered form inside the ferrite grains, as it can be seen in Fig. 12. Comparing the images in Fig. 12 and 7 it can be seen that the pits nucleated at the nitride precipitation sites. This is because of the lower chromium and nitrogen content around the nitride precipitations, which locally decreases the pitting corrosion resistance (Ref 49,50,51), representing the lowest Epit value among the investigated samples. The samples with Δt12/8 = 50 s cooling time show a similar pitting mechanism as the as-received state. The pits are initiated in the ferrite grains, while the intergranular, partially transformed, and grain boundary austenite grains remain intact. It can also be observed crevice corrosion was avoided with the proper sample preparation steps, as the ~ 10 µm thick austenitic boundary layer at the edges has not gone under corrosion degradation during the tests.
4 Conclusions
In this paper, the Gleeble physically simulated heat-affected zones (HAZs) of lean duplex grade X2CrNiN22-2 were investigated. From the results, the following conclusions can be drawn:
-
The shorter cooling times show more ferritic, the longer cooling times more austenitic microstructures. In the more rapidly cooled samples thin plates of grain boundary austenite, with increasing cooling times also intergranular and Widmanstätten-type austenite is visible.
-
The increasing cooling time means increasing grain boundary austenite plate width (GBAW). In the sample with Δt12/8 = 1 s cooling time, the average GBAW is 16 ± 1 µm, which increases to 26 ± 3 µm after 12 s, and 37 ± 6 µm after 50 s cooling time.
-
The oxalic acid electrochemical etching in the highly ferritic HAZ microstructures shows nitride precipitations inside the ferrite grains.
-
The austenite content in the HAZs can be described by the following equation:
$$A\% = 30 \cdot \Delta t_{12/8}^{0.06} ,\;\left( \% \right)$$
Regarding weldability, the HAZ of the grade 2202 shows an excellent behavior as even at shorter cooling times (lower heat inputs) the austenite content remains above 30% in average.
-
The different cooling times significantly affect the corrosion resistance properties of lean duplex grade 2202. The Ecorr values varied in the range of − 65 ± 12 mV (as-received sample) to − 241 mV ± 24 (50 s cooling time) vs. the saturated Ag/AgCl (KCl sat.) reference electrode in 3.5 wt.% NaCl solution at 25 °C.
-
Regarding the pitting corrosion mechanisms, the simulated HAZ microstructures show reduced Epit values, meaning lower resistance against pitting corrosion. The average Epit values for Δt12/8 = 1 s are 573 ± 31 mV, for 12 s 706 ± 41 mV, and for 50 s 670 ± 33 mV. The lowest pitting potential was measured in the case of the most rapidly cooled sample, where the nitride precipitation acts as a nucleation site.
References
M. Theofanous and L. Gardner, Experimental and Numerical Studies of Lean Duplex Stainless Steel Beams, J. Constr. Steel Res., 2010, 66, p 816–825. https://doi.org/10.1016/j.jcsr.2010.01.012
N. Saliba and L. Gardner, Cross-Section Stability of Lean Duplex Stainless Steel Welded I-Sections, J. Constr. Steel Res., 2013, 80, p 1–14. https://doi.org/10.1016/j.jcsr.2012.09.007
D.M. Kemény and D. Kovács, The Effect of Welding Parameters on the Corrosion Resistance of Austenitic Stainless Steel, Periodica Polytechnica, Mech. Eng., 2022, 66, p 151–157. https://doi.org/10.3311/PPme.19568
A.S. Alcantara, E.R. Fábián, M. Furkó, É. Fazakas, J. Dobránszky, and T. Berecz, Corrosion Resistance of TIG Welded Joints of Stainless Steels, Mater. Sci. Forum, 2017, 885, p 190–195. https://doi.org/10.4028/www.scientific.net/MSF.885.190
E. Kalácska, K. Májlinger, E.R. Fábián, and P. Russo Spena, MIG-Welding of Dissimilar Advanced High Strength Steel Sheets, Mater. Sci. Forum, 2017, 885, p 80–85. https://doi.org/10.4028/www.scientific.net/MSF.885.80
K.U. Rani, R. Kumar, M.M. Mahapatra, R.S. Mulik, A. Świerczyńska, D. Fydrych, and C. Pandey, Wire Arc Additive Manufactured Mild Steel and Austenitic Stainless Steel Components: Microstructure, Mech. Prop. Residual Stress., Mater., 2022, 15, p 7094. https://doi.org/10.3390/ma15207094
E.A. Ferreira, R.C. Silva, L.A. Costa, C.P. de Oliveira, G.C. Silva, A.D. Paula, C.S. Fugivara, and A.V. Benedetti, Is Duplex Stainless Steel More Corrosion Resistant Than 316L in Aqueous Acid Chloride-Containing Environments at Temperatures Higher Than 100°C?, Corros. Eng. Sci. Technol., 2018, 53, p 502–509. https://doi.org/10.1080/1478422X.2018.1507335
S. Aribo, R. Barker, X. Hu, and A. Neville, Erosion–Corrosion Behaviour of Lean Duplex Stainless Steels in 3.5% NaCl Solution, Wear, 2013, 302, p 1602–1608. https://doi.org/10.1016/j.wear.2012.12.007
E.A. de Pauli, F. Cotting, J.C.S. Junior, I.V. Aoki, and S.D. Brandi, Welding Heat Input Influence on UNS S82441 Lean Duplex Stainless Steel Corrosion Resistance Assessed by Scanning Vibrating Electrode Technique (SVET), J. Mater. Eng. Perform., 2018, 27, p 6389–6397. https://doi.org/10.1007/s11665-018-3721-z
M. Liljas, P. Johansson, H.-P. Liu, and C.-O.A. Olsson, Development of a Lean Duplex Stainless Steel, Steel Res. Int. Res. Int., 2008, 79, p 466–473. https://doi.org/10.1002/srin.200806154
J. Peultier, S. Jacques, and V. Baudu, Lean Duplex UR2202: An Alternative to 304L with Increased Corrosion Resistance and Mechanical Properties, Revue de Métall., 2009, 106, p 214–219. https://doi.org/10.1051/metal/2009036
M. Snis and J. Olsson, Reduce Costs for Storage and Distribution of Desalted Water—Use Duplex Stainless Steel, Desalination, 2008, 223, p 476–486. https://doi.org/10.1016/j.desal.2007.04.068
R. Francis and G. Byrne, Duplex Stainless Steels—Alloys for the 21st Century, Metals (Basel)., 2021, 11, p 836. https://doi.org/10.3390/met11050836
M. Dalal and J. Penso, Duplex Stainless Steel—Learning From Field Experience in Oil & Gas and Petrochemical Services, in Volume 4B: Materials and Fabrication, American Society of Mechanical Engineers, 2022. https://doi.org/10.1115/PVP2022-84889
T. Kim, S. Hong, B. Hwang, and J. Kim, Block Shear Capacity in Cold-Formed Lean Duplex Stainless Steel Double-Shear Bolted Connections, Thin-Walled Struct., 2021, 161, p 107520. https://doi.org/10.1016/j.tws.2021.107520
Y. Huang and B. Young, Finite Element Analysis of Cold-Formed Lean Duplex Stainless Steel Columns at Elevated Temperatures, Thin-Walled Struct., 2019, 143, p 106203. https://doi.org/10.1016/j.tws.2019.106203
L. Pezzato and I. Calliari, Advances in Duplex Stainless Steels, Materials, 2022, 15, p 7132. https://doi.org/10.3390/ma15207132
A.N. Chaudhari, K. Dixit, G.S. Bhatia, B. Singh, P. Singhal, and K.K. Saxena, Welding Behaviour of Duplex Stainless Steel AISI 2205: A Review, Mater. Today Proc., 2019, 18, p 2731–2737. https://doi.org/10.1016/j.matpr.2019.07.136
E.M. Westin, Hot Cracking in Duplex Stainless Steel Weldments—A Review, Weld. World, 2022, 66, p 1483–1499. https://doi.org/10.1007/s40194-022-01310-8
E.J. da Cruz Junior, J. Gallego, A.G. Settimi, C. Gennari, A. Zambon, and V.A. Ventrella, Influence of Nickel on the Microstructure, Mechanical Properties, and Corrosion Resistance of Laser-Welded Super-Duplex Stainless Steel, J. Mater. Eng. Perform., 2021, 30, p 3024–3032. https://doi.org/10.1007/s11665-021-05590-x
T.-H. Pham and N.-V. Nguyen, Mechanical Properties of Constituent Phases in Structural Steels and Heat-Affected Zones Investigated by Statistical Nanoindentation Analysis, Constr. Build. Mater., 2021, 268, p 121211. https://doi.org/10.1016/j.conbuildmat.2020.121211
J. Łabanowski, T. Rzychoń, W. Simka, and J. Michalska, Sulfate-Reducing Bacteria-Assisted Hydrogen-Induced Stress Cracking of 2205 Duplex Stainless Steels, Mater. Corros. Corros., 2019, 70, p 1667–1681. https://doi.org/10.1002/maco.201910802
C.R. de Farias Azevedo, H. Boschetti Pereira, S. Wolynec, and A.F. Padilha, An Overview of the Recurrent Failures of Duplex Stainless Steels, Eng. Fail. Anal., 2019, 97, p 161–188. https://doi.org/10.1016/j.engfailanal.2018.12.009
S.M. Alvarez, A. Bautista, and F. Velasco, Corrosion Behaviour of Corrugated Lean Duplex Stainless Steels in Simulated Concrete Pore Solutions, Corros. Sci.. Sci., 2011, 53, p 1748–1755. https://doi.org/10.1016/j.corsci.2011.01.050
L. Zhang, W. Zhang, Y. Jiang, B. Deng, D. Sun, and J. Li, Influence of Annealing Treatment on the Corrosion Resistance of Lean Duplex Stainless Steel 2101, Electrochim. Acta. Acta, 2009, 54, p 5387–5392. https://doi.org/10.1016/j.electacta.2009.04.023
C. Örnek, F. Léonard, S.A. McDonald, A. Prajapati, P.J. Withers, and D.L. Engelberg, Time-Dependent In Situ Measurement of Atmospheric Corrosion Rates of Duplex Stainless Steel Wires, Npj Mater. Degrad., 2018, 2, p 10. https://doi.org/10.1038/s41529-018-0030-9
L.H. Guilherme, P. Reccagni, A.V. Benedetti, C.S. Fugivara, and D.L. Engelberg, Corrosion Assessment of ASME Qualified Welding Procedures for Grade 2101 Lean Duplex Stainless Steel, Corrosion, 2019, 75, p 1216–1229. https://doi.org/10.5006/3257
L.H. Guilherme, A.V. Benedetti, and C.S. Fugivara, A Portable Electrochemical Microcell for Weld Inspection of Duplex Stainless Steel Tanks, Corrosion, 2019, 75, p 340–348. https://doi.org/10.5006/3004
Y. Yang and X. Pan, Effect of Mn/N Ratio on Microstructure and Mechanical Behavior of Simulated Welding Heat-Affected Zone in 22% Cr Lean Duplex Stainless Steel, Mater. Sci. Eng. A, 2022, 835, p 142676. https://doi.org/10.1016/j.msea.2022.142676
T. Wu, J. Wang, H. Li, Z. Jiang, C. Liu, and H. Zhang, Effect of Heat Input on Austenite Microstructural Evolution of Simulated Heat-Affected Zone in 2205 Duplex Stainless Steel, J. Iron. Steel Res. Int., 2019, 26, p 435–441. https://doi.org/10.1007/s42243-018-0134-z
L. Pezzato, M. Lago, K. Brunelli, M. Breda, and I. Calliari, Effect of the Heat Treatment on the Corrosion Resistance of Duplex Stainless Steels, J. Mater. Eng. Perform., 2018, 27, p 3859–3868. https://doi.org/10.1007/s11665-018-3408-5
A.E. Odermatt, V. Ventzke, F. Dorn, R. Dinsé, P. Merhof, and N. Kashaev, Effect of Laser Beam Welding on Microstructure, Tensile Strength and Fatigue Behaviour of Duplex Stainless Steel 2205, J. Manuf. Process., 2021, 72, p 148–158. https://doi.org/10.1016/j.jmapro.2021.10.020
T. Tóth, J. Hensel, A.-C. Hesse, and K. Dilger, Electron Beam Welding of 2205 Duplex Stainless Steel with Nickel-Based Filler Wire Using Multi-Beam Technique, Weld. World, 2022, 66, p 2191–2206. https://doi.org/10.1007/s40194-022-01363-9
C. Gennari, M. Lago, B. Bögre, I. Meszaros, I. Calliari, and L. Pezzato, Microstructural and Corrosion Properties of Cold Rolled Laser Welded UNS S32750 Duplex Stainless Steel, Metals (Basel), 2018, 8, p 1074. https://doi.org/10.3390/met8121074
R.-I. Hsieh, H.-Y. Liou, and Y.-T. Pan, Effects of Cooling Time and Alloying Elements on the Microstructure of the Gleeble-Simulated Heat-Affected Zone of 22% Cr Duplex Stainless Steels, J. Mater. Eng. Perform., 2001, 10, p 526–536. https://doi.org/10.1361/105994901770344665
P. Reccagni, L.H. Guilherme, Q. Lu, M.F. Gittos, and D.L. Engelberg, Reduction of Austenite-Ferrite Galvanic Activity in the Heat-Affected Zone of a Gleeble-Simulated Grade 2205 Duplex Stainless Steel Weld, Corros. Sci.. Sci., 2019, 161, p 108198. https://doi.org/10.1016/j.corsci.2019.108198
Y. Guo, T. Sun, J. Hu, Y. Jiang, L. Jiang, J. Li, Y. Guo, T. Sun, H. Jincheng, Y. Jiang, L. Jiang, and J. Li, Microstructure Evolution and Pitting Corrosion Resistance of the Gleeble-Simulated Heat-Affected Zone of a Newly Developed Lean Duplex Stainless Steel 2002, J. Alloys Compd., 2016, 658, p 1031–1040. https://doi.org/10.1016/j.jallcom.2015.10.218
B. Varbai, Y. Adonyi, R. Baumer, T. Pickle, J. Dobránszky, and K. Májlinger, Weldability of Duplex Stainless Steels—Thermal Cycle and Nitrogen Effects, Weld. J., 2019, 98, p 78–87. https://doi.org/10.29391/2019.98.006
S. Hertzman, P.J. Ferreira, and B. Brolund, An Experimental and Theoretical Study of Heat-Affected Zone Austenite Reformation in Three Duplex Stainless Steels, Metall. Mater. Trans. A, 1997, 28, p 277–285. https://doi.org/10.1007/s11661-997-0130-6
L. Karlsson, Welding Duplex Stainless Steels—A Review of Current Recommendations, Weld. World, 2012, 56, p 1–17. https://doi.org/10.1007/BF03321351
G.F. Vander Voort, Metallogr. Microstruct. (2004)
B. Varbai and K. Májlinger, Optimal Etching Sequence for Austenite to Ferrite Ratio Evaluation of Two Lean Duplex Stainless Steel Weldments, Measurement, 2019, 147, p 106832. https://doi.org/10.1016/j.measurement.2019.07.060
ISO 17781:2017, Petroleum, Petrochemical and Natural Gas Industries—Test Methods for Quality Control of Microstructure of Ferritic/Austenitic (duplex) Stainless Steels. (2017)
W. Zhang, T. DebRoy, T.A. Palmer, and J.W. Elmer, Modeling of Ferrite Formation in a Duplex Stainless Steel Weld Considering Non-Uniform Starting Microstructure, Acta Mater. Mater., 2005, 53, p 4441–4453. https://doi.org/10.1016/j.actamat.2005.05.040
H. Matsunaga, Y.S. Sato, H. Kokawa, and T. Kuwana, Effect of Nitrogen on Corrosion Of Duplex Stainless Steel Weld Metal, Sci. Technol. Weld. Join., 1998, 3, p 225–232. https://doi.org/10.1179/stw.1998.3.5.225
J.C. Lippold and D.J. Kotecki, Welding Metallurgy and Weldability of Stainless Steels, Wiley, Hoboken, (2005)
M. Ito, K. Ohashi, K. Fujii, K. Hombo, K. Miyazaki, A. Ooi, E. Tada, and A. Nishikata, Identification of Chromium-Depleted Area around Chromium Nitride Precipitates in Heat-affected Zone of Lean-Duplex Stainless Steel and In-situ Observation of Preferential Dissolution by EC-AFM, ISIJ International. 62 (2022) ISIJINT-2021-462. doi:https://doi.org/10.2355/isijinternational.ISIJINT-2021-462
E.M. Westin, B. Brolund, and S. Hertzman, Weldability Aspects of a Newly Developed Duplex Stainless Steel LDX 2101, Steel Res. Int., 2008, 79, p 473–481.
S. Cui, Y. Shi, Y. Cui, and T. Zhu, The Influence of Microstructure and Chromium Nitride Precipitations on the Mechanical and Intergranular Corrosion Properties of K-TIG Weld Metals, Constr. Build. Mater., 2019, 210, p 71–77. https://doi.org/10.1016/j.conbuildmat.2019.03.212
J.C. de Lacerda, L.C. Cândido, and L.B. Godefroid, Corrosion Behavior of UNS S31803 Steel with Changes in the Volume Fraction of Ferrite and the Presence of Chromium Nitride, Mater. Sci. Eng. A, 2015, 648, p 428–435. https://doi.org/10.1016/j.msea.2015.09.092
S. Geng, J. Sun, L. Guo, and H. Wang, Evolution of Microstructure and Corrosion Behavior in 2205 Duplex Stainless Steel GTA-Welding Joint, J. Manuf. Process., 2015, 19, p 32–37. https://doi.org/10.1016/j.jmapro.2015.03.009
Acknowledgment
This paper has been supported by the National Research, Development and Innovation Office – NKFIH, OTKA PD 138729. Project no. TKP-6-6/PALY-2021 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NVA funding scheme. Special thanks to Marcell Gáspár and Raghawendra Sisodia from the University of Miskolc for performing the Gleeble physical heat-affected zone simulations. Thanks to Dorina Kovács from Budapest University of Technology and Economic for her help taking the SEM images. Thanks to Aperam Stainless Europe for providing the 2202 samples for my research.
Funding
Open access funding provided by Budapest University of Technology and Economics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varbai, B. Heat-Affected Zone Characterization of X2CrNiN22-2 Lean Duplex Stainless Steel by Metallographic and Electrochemical Techniques. J. of Materi Eng and Perform 32, 11240–11249 (2023). https://doi.org/10.1007/s11665-023-08807-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08807-3