Abstract
Samples based on 316L stainless steel were prepared by conventional manufacturing process (CM) and laser powder bed fusion (L-PBF). Surface morphology changes under air oxidation in the temperature range 600-900 °C were carried out. Tensile tests were carried out in the temperature range of 700-900 °C for strain rates between 0.001 and 0.1 s−1. The materials showed good oxidation resistance up to 700 °C. The CM and L-PBF material had a high mass gain instability and similar microstructures developed under high temperatures were found in both alloys. Increased temperature increases Cr concertation in the L-PBF material up to 40 at.% at 800 °C and a rich Fe based oxide is formed at 900 °C. Slightly thicker oxide scales were formed in the CM than in the L-PBF material.The hot tensile tests reveal that a fast work hardening occurs for all hot tensile tested samples up to a strain of approximately 0.025. Low temperatures and high strain rates within the investigated range promote a second work hardening regime, while a plateau in the flow stress is observed at high temperatures and low strain rates. The highest yield stress and peak stress values are reached at 700 °C. The yield stress is nearly independent of the strain rate at 700 °C. It decreases with a decrease in strain rate for 800 and 900 °C, and it decreases with an increase in temperature. The elongation till fracture varies from 10 to 22%, and it is strongly influenced by defects inherent of the L-PBF process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-temperature corrosion is critical in many industrial sections. Oxidation, sulfidation and nitration are the main high-temperature mechanisms of corrosion (Ref 1). Stainless steels (SS) are commonly used in high-temperature applications due to their generally good oxidation resistance and economic advantages (Ref 1, 2). For example, 316L stainless steel is used in various applications due to its high mechanical strength and generally good oxidation resistance at high temperatures. However, the extensive oxide spallation of this material may reduce its lifespan and potential use when submitted to too high-temperature environments. Various industrial sectors are interested in additive manufacturing (AM) because of its advantages compared with conventional subtractive-based manufacturing (Ref 3,4,5). Several AM practices have been applied to various materials (Ref 5). Laser powder bed fusion (L-PBF) AM has drawn attention since it can produce complex shapes compared with conventional technologies (Ref 6, 7). L-PBF offers excellent opportunities for the increasing demand for an individual, resource-efficient production of complex parts. Studies on the high-temperature oxidation of stainless steel can be found in (Ref 8,9,10,11,12,13,14,15,16,17,18). In the case of stainless steel fabricated via powder metallurgy, the resistance to oxidation and the oxide's chemical composition are strongly influenced by the porosity of the material (Ref 12, 13, 19). A double oxide film of Cr2O3 and Fe2O3 oxide particles in the outer and FeCr2O4 oxide in the inner is observed in a wrought 316L after air oxidation at 400 °C (Ref 20). A critical exposure period of around 100 h exists in the air oxidation process at 60 °C for the wrought 316L (Ref 20). A compact oxide film decorated with oxide particles transforms into a loose oxide layer with a pore structure (Ref 20). For longer exposures at 600 °C, a double oxide film containing a Fe-rich outer oxide layer and a Cr-rich inner oxide layer is formed (Ref 20). The thickness increases over exposure periods (Ref 20). Similar results are found for a 316L produced by L-PBF (Ref 21). Fe2O3 is the dominant oxide for 650, 700 and 750 °C when a wrought 316L is exposed to air (Ref 10). The oxide layer consists of a homogeneous part at 650 °C (Ref 10). A double oxide layer is formed at 700 and 750 °C (Ref 10). When exposed to air at 800 °C, an inhomogeneous elemental distribution of Cr and Fe during the early stages of oxidation occurs for a wrought 316L alloy (Ref 22). Each oxide grain consists of consecutive Cr-rich and Fe-rich layers parallel to the surface (Ref 22). Spallation of the oxide scale occurs at 800 °C regardless of exposure periods, resulting in different oxidation morphologies, and the degree of spallation behavior worsens with the increase in exposure time (Ref 20). While a Cr depletion at the surface of a wrought 316L alloy is observed after exposure to air at 800 °C, the Cr supply was insured in the case of an L-PBF fabricated 316L alloy (Ref 23).
The suppression of large defects in L-PBF 316L materials can significantly increase ductility (Ref 24,25,26,27). The desirable combination of strength and ductility is achievable for LPBF 316L at room temperature (Ref 28, 29). These superior properties were attributed to specific microstructural features at different length scales, particularly the presence of fine cellular structures. Dryepondt et al. (Ref 30) investigated the influence of annealing on microstructure and high-temperature tensile properties of 316L fabricated by L-BPF. The cellular structure is stable up to 500 °C, but yield strength decreases above 600 °C. The effect of nanoparticles on the strengthening at low and high temperatures in the 316L produced by L-PBF was also investigated (Ref 31, 32). Dynamic recovery and discontinuous dynamic recrystallization occurred at 700 and 800 °C (Ref 32).
This work investigates the oxidation and tensile behavior of an L-PBF-produced 316L alloy at high temperatures. Specifically, this work describes the differences in oxidation behavior, microstructure development and oxide scale thickness in dry air between a commercial wrought stainless steel and an L-PBF-produced one in a temperature range of 600-900 °C. In addition, the hot tensile behavior of the L-PBF produced is also investigated to determine the influence of temperature and strain rate on the strength and elongation to fracture.
2 Experimental Procedure
2.1 Sample Preparation
Austenitic chromium-nickel-molybdenum 316L SS available on the market was used as a thin sheet. The 316L SS for air oxidation tests at high temperatures in the form of sheet was bought and cut for small squares 10 × 10 × 2 mm. The chemical analysis of the samples (average value from 5 different EDS spot analysis) prior oxidation study is shown in Table 1.
The L-PBF additive manufacturing process produced the other material used in oxidation and tensile tests. A standard 316L powder from Carpenter Additive Manufacturing was fabricated according to the ASTM F3184 and used in this work. Table 2 shows chemical composition of the powder used to print jobs.
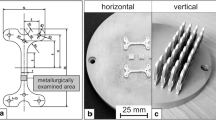
A Coherent Creator RA 3D metal printer was used to produce tensile samples using a laser power of 20 W, a scan speed of 1000 mm/s, a layer height of 25 µm and a hatching distance of 55 µm. The L-PBF printed 316L material was used as small coupons (cylinders of 10 mm in diameter × 2 mm in thickness). Before air oxidation tests, both types of 316L material were ground with 600 SiC paper to achieve similar surface roughness. Then, the samples were cleaned in acetone using an ultrasonic bath at 40 °C for 15 min and dried using cool air at room temperature. This process was carried out to remove any impurities from the sample surfaces.
2.2 Oxidation Testing
Air oxidation tests were carried out for 100 h in the temperature range of 600-900 °C. A tubular furnace was used for the oxidation tests of the 316L CM and 316L L-PBF samples. The rig used a SiO2 tube with an Al2O3 double deck holder for the samples. The ramp rate in the oxidation process was set up for 5 °C/min to prevent the corundum tube from failing. The samples were placed on an inert Al2O3 ceramic holder within the hot zone of the furnace. The hot zone was calibrated using thermocouple K. The length of the hot zone was 10 cm. The SiO2 tube was closed from both sides using ceramic plugs to avoid laboratory air enter to a hot rig. Every 25 h, scanning electron microscopy (SEM) images in secondary (SE) and backscattered electron (BSE) modes were captured to assess microstructural changes and oxide development in the samples. Figure 1 shows a schematic drawing of the air oxidation rig used in this work.
2.3 Hot Tensile Testing
The L-PBF SS was tensile tested at high temperatures. The tensile test samples were tested in the as-built condition without any surface machining. The tensile samples had a gauge section of 5 mm in diameter and 25 mm in length following the EN 10,025 standard. The tests were carried out at 700 and 800 °C for the strain rates of: 0.001, 0.01 and 0.1 s−1. In addition, the L-PBF SS was also tested at 900 °C for the strain rates of: 0.001 and 0.01 s−1. Each individual sample was heated up within the furnace and kept for 5 min prior to deformation. The tensile tests were carried out on Schenck/Instron 8800 M machine using Bluehill software and Maytec HTO-09 high-temperature rig.
2.4 Microstructure Characterization
The surface of the oxidized samples was analyzed using a Tescan Mira3 scanning electron microscope (SEM), and secondary electrons (SE), as well as backscattered electrons (BSE) micrographs, were acquired. A voltage of 15 kV, a spot size of 20 nm and a work distance from 10 to 15 mm were used for the SEM analysis of the surface of the oxidized samples. The cross sections of the oxidized samples were also investigated. First, the samples were cut into their cross sections, embedded into resin, mechanically ground and polished. The oxide scale was investigated using a Scios 2 DualBeam FEI SEM, and the chemical composition was semi-quantitatively assessed using energy-dispersive x-ray spectroscopy (EDS). During the surface analyses, a voltage of 20 kV and a work distance of 6.4 mm were used for the SEM analysis of the surface of the oxidized samples. The polished cross sections were etched using aqua regia solution to assess changes in microstructure upon oxidation, a mixture of nitric acid and hydrochloric acid in a molar ratio of 1:3. Finally, light micrographs of the bulk material were acquired using Carl Zeiss AxioObserver Z1m.
3 Results and Discussion
3.1 Surface Investigations
The microstructures of the bulk of the 316L CM SS subjected to oxidation are shown in Fig. 2. In all cases, a recrystallized microstructure is shown, and grain coarsening does not seem pronounced even after 100 h heat treatment at high temperature.
Figure 3 shows the bulk microstructures of the 316L AM SS material after oxidation for 100 h. Overall, a microstructure with low porosity is produced. A blocky austenitic macrostructure is shown after 100 h exposure at 600 °C, Fig. 3(A) resultant of the LPB-F process. With an increase in temperature, the blocky grains start to recrystallize statically. However, even after 100 h at 900 °C, the melt pool lines are still visible. It indicates that the stored energy for microstructural changes upon heat treatment is small for the initial 316L AM SS material.
Figure 4 shows the surface microstructure of 316L CM SS developed in the temperature range 600-900 °C for 100 h, respectively.
Figure 5 shows the SEM images in SE mode for the oxide scale development process on austenitic steel exposed to air. At the lowest temperature (600 °C), the exposed surface developed randomly distributed crystals with the size of a few nanometers. Nevertheless, not a whole surface was covered at 600 °C with tiny crystals. Instead, some areas developed a Fe-rich oxide, as shown in Fig. 5.
The EDS analyses in Fe-rich region confirmed: 43.1 Fe, 46.0 O, 2.2 Cr, 0.8 Mn and finally 1.7 at.% Si. No other elements were shown during this analysis. Fe-rich oxide in austenitic steel in the air is natural since the steel is not uniform in terms of chemical composition. Some places in the matrix are enriched in Fe and develop non-protective oxides based on Fe under highly oxidizing conditions. On the other hand, the formation of tiny enriched crystals spread on the surface is shown. The detailed EDS analyses of the crystals revealed a chemical composition of 16.4 O, 0.9 Si, 0.7 Mo, 13 Cr, 4.3 Mn, 54.0 Fe and finally 7.9 at.% Ni. This composition is similar to the metal matrix of 316L grade but with O content. The matured crystals on the 316L surface are too small, and the EDX measurement mainly reveals the bulk composition.
The surface observations after the exposure at 700 °C shown in Fig. 4(B) indicate the presence of slightly bigger crystals than at 600 °C (Fig. 4A). The crystals were more uniformly distributed on the 316L steel surface and covered the surface to a higher degree. The average chemical composition based on EDS analysis of the crystals showed the following results: 41.0 O, 18.5 Cr, 8.2 Mn, 3.9 Ni and finally 25.5 at.% Fe, the other elements Si. EDS with less than 1 at.% detected Mo in total. A higher temperature (800 °C) of the exposure dominated the formation of rich Cr crystals with as high as: 44.3 O, 5.6 Fe, 40.3 Cr, 6.3 Mn, 1.2 at.% Ni, the Si content was lower than 1 at.%, no Mo found by EDS analyses. A higher temperature indicates a higher diffusion flux and a higher Cr concentration in the oxide scale. The Cr diffusion from the metal matrix privileges forming Cr-rich crystals due to its high centration and much lower Gibbs free energy formation (Ref 33). Therefore, the formation of Cr-rich oxide scale at 800 °C is preferred to the formation of Fe-rich oxide scale, as was shown at 600 °C. The exposure of the 316L sample at 900 °C for 100 h formed a mixed oxide scale with different microstructures, as shown in Fig. 6.
Figure 6(A) shows an oxide structure with the following chemical composition: 45.3 O, 23.8 Cr, 29.5 Fe and 1.5 at.% Ni, no other elements were found. The composition detected indicates the formation of a spinel such as FeCr2O4. The spinel forms through reactions in solid at high temperatures, using Fe3O4 and Cr2O3 (Ref 34). Some parts of the surface of the exposed sample at 900 °C were covered by larger crystals with a similar composition as found at 800 °C, as Fig. 6(B) shows. The exposure at higher temperatures facilitates diffusion of Mn from the metal matrix in the same way as Cr, mixed oxide scale composed with MnCr2O4 and some fractions of FeCr2O4 spinel (Ref 20, 34).
Figure 7 shows the surface microstructure of 316L AM SS developed at 600-900 °C for 100 h. The microstructures developed in 316L AM SS materials are similar to that developed in 316L CM SS at 600, 700 and 800 °C. Generally, the surface is covered by crystals and the higher temperature, the larger the crystals. However, at 900 °C, the crystals formed in 316L AM SS were notably bigger than those in the 316L CM SS sample.
Chemical composition evaluated by EDS on 316L AM SS material reveals that at 600 °C, the composition of the oxide scale is similar to 316L CM SS material. Due to the formation of tiny crystals, the analyses showed metal matrix elements such as Si, Mo, and Ni. The main contributors of the oxide scale were related to: O: 29.4, Cr:13.5 and finally Fe: 43.3 at.%. The EDS analyses performed on 8 different spots from time to time showed Mn with different concentrations: 3.6 at.%. At 700 °C, the EDS analyses from 8 spots showed high Cr: 22.4 and Mn 14.5 at.% contents compared to the EDS analyses from 600 °C exposure. The other elements remained with a similar concentration than was observed at 600 °C. Oxidation at 800 °C in the air formed oxide crystals rich in Cr: 25.at.%, Mn: 22 at.% and O: 48.3 at.%. The other elements, such as Fe and Si, reached as high as 2-3 at.%. Mo was found in different surface places with concentrations lower than 1 at.%. Finally, the exposure of 316L CA SS material at 900 °C forms oxides with over 30 Cr and 17 at.% Mn. Such patches were randomly distributed across the surface. Other regions of the oxide scale consisted of Cr and Mn, Fe and Ni with as low as 2.5 and 1.2 at.%, respectively. The results indicate that the higher the temperature, the higher the Cr diffusion to the oxide scale, which is observed together with Mn that supports the formation of a protective oxide scale. The presence of Mn and the formation of spinel such as MnCr2O4 are beneficial for the materials exposed to high temperatures. Sachitanand et al. (Ref 35) reported clear evidence of a relationship between high Mn content in the formed oxide scale. Lower Cr content is needed to form a protective scale when Mn content is high enough. However, high Mn concentration in the oxide scale may lead to a higher mass gain and the formation of a thicker MnCr2O4 spinel layer. Holcomb et al. (Ref 36) found that Mn additions to Cr decrease the activity of chromium within the oxide, due to the solid solution replacement of chromium with manganese (at low levels of manganese). Another study presented by Yang et al. (Ref 37) found that the presence of Mn facilitated the formation of a bi-layered oxide scale, where an outer consisted M3O4 (M = Mn, Cr, Ni) spinel-rich layer at the oxide–gas interface over a Cr2O3-rich sub-layer at the metal–oxide interface.
3.2 Cross Section Investigations
Figure 8(A)-(D) shows the commercially manufactured 316L SS cross sections after the exposure for 100 h at 600-900 °C, respectively.
The exposure of 316L CM SS at the lowest temperature showed the development of the thickest oxide scale on the corners, whereas a very thin oxide layer covered the flat surface of the samples. The oxide scale developed on the surface was double structured with a slightly thicker outer layer than the inner layer, and the oxide scale was slightly porous. Chemical analyses of the oxide were performed using EDS line scan mode, as shown in Fig. 8(A) that confirms the double-layered oxide scale formation. It clearly shows four different regions. The region designated with number 1 is localized in the outer part of the scale with a high O content: 55, Fe: 40 at.% a low content of Cr: 5 at.% and finally marginal content Mn, Ni and finally Si. This region shows the development of rich Fe oxide with additional Cr content. However, this Cr content was much lower than this mentioned in this work previously when surface chemical composition development at 900 °C was given. Region 2, with a thickness of 1.3 µm, within the oxide scale, was enriched in Cr: 30 with a low Fe content 17 and Ni 5 at.%. The other elements in this region were in low concentration (below 2 at.%). The final layer in the oxide scale, labeled as region 3, with a thickness similar to the outermost layer (region 1), was rich in Fe: 25 and Cr: 18 at.%. The concentration of Cr was much higher in this region than in the outermost region. The Ni concentration was enriched toward the oxide scale–substrate interface. The final region marked as 4 is related to the metal matrix of 316L SS. The oxide scale of 316L CM SS that formed at 700 °C in the air for 100 h is shown in Fig. 8(B) on SEM image with corresponding EDS scan line. Compared to the oxide scale formed at a lower temperature, the thickness of the oxide scale is similar. At 700 °C, the scale consisted of 4 different layers. The outermost layer, 6 µm thick, consisted of Fe: 50, O: 45 and other elements with a total concentration of 5 at.%, and the middle layer was enriched with Cr and Ni. However, this layer can be further divided into sub-layers. The upper part of this layer was enriched in Cr: 17 and Ni: 10 with Fe: 20 and as high as: 50 at.% O. The bottom part of this layer was further enriched in Cr (up to 30 at.%), lean in Ni and Fe, and with constant O concentration (50 at.%). The highest Cr content was observed in layer 3, where EDS analysis detected almost 50 at.%. This layer was very thin (1 µm). The final region observed in the EDS scan line reveals the metal matrix. The observed results showed that the main contributors in oxidation behavior are related to the formation of Fe oxide in the outermost layer and the formation of the mixture of oxides with different concentrations of Ni, Cr, Fe and O. The elements Si, Mn are found to be in marginal concentration across the whole EDS line scan.
The SEM BSE mode image of the oxide scale developed at 800 °C for 100 h with the corresponding EDS scan line of 316L CM SS is shown in Fig. 8(C). A relatively thick scale that was used for chemical analysis was developed on the edge of the sample. The total thickness of the scale reached 7 µm. A very thin oxide scale covered the other part of the material with a total thickness of less than 1 µm. In concentration profiles, the outermost region (region 1) was enriched in Fe: 28, O: 52 and Cr: 18 at.%. However, the inner part of region 1 shows lowered concentration of Cr: 5, increased Fe concentration: 40 and constant O content: 50 at.%, the bottom part of region 1 again showed a higher concentration of Cr: 20 and lowered amount of Fe (20 at.%). The middle part of the oxide scale (region 2) was enriched in Cr: 33 and lowered concentration of Fe: 18, O: 45 at.% was found.
The cross section SEM image of the 316L CM SS exposed at 900 °C for 100 h, and the corresponding EDS line scan is shown in Fig. 8(D). The oxide scale formed at 900 °C has a porous multiphase structure with the development of different oxides. In contrast to the exposures at lower temperatures, there is no thick scale consisting of a single or double-layer structure. However, there are several layers with enriched Ni concentration and alternative layers with Cr and Fe enrichments. The porous sandwich-like structure developed a 15 µm oxide scale. Similar to the exposures at lower temperatures, Si and Mn were found with marginal concentration.
Figure 9 shows the cross section images of the 316L AM SS samples exposed at 600-900 °C for 100 h with corresponding EDS line scans. The SEM images were captured in lower and higher magnifications to show overall oxidation behavior and specify the area for EDS scan line analysis.
The analyses performed on the oxidized samples clearly show the impact of temperature on oxidation behavior. At 600 °C (Fig. 9A), the 316L AM SS material showed good oxidation resistance behavior, forming a thin oxide scale similar to the one formed in the 316L CM SS material. According to EDS line scan analysis, the main elements in the oxide scale are: Fe, Cr, and O. Those elements are favorable to form a mixed oxide scale in the rich oxygen atmosphere. The detectable oxide scale reached 0.4 µm, whereas the observable diffusion zone where oxygen penetrated the metal matrix reached 1.0 µm (black arrow in EDS concentration profiles). In contrast to 316L CM SS, the exposed material formed a much thinner oxide scale. However, similar elements contributed to the oxide scale formation. At a slightly higher temperature (700 °C), the oxide scale's thickness was similar to that observed at 600 °C, as Fig. 9(B) shows. The results indicate that the 316L AM SS has good oxidation protection. The corresponding EDS analysis of the sample exposed at 700 °C showed a high Cr concentration. The Cr concentration reached 20 at.% at 700 °C (at 600 °C was 12 at.%). This behavior shows that higher temperatures promote a higher diffusion of Cr. A higher concentration of Cr in the oxide scale improves the oxidation resistance. The trend was also observed at 800 °C (Fig. 9C), where the concentration of Cr in the oxide scale reached values as high as 40 at.%. Furthermore, at 800 °C, an enriched Si zone was observed underneath the oxide scale. The formation of SiO2 underneath the oxide scale is an additional diffusion barrier for O diffuse inwardly toward the metal matrix. Nowak pointed out the formulation of SiO2 in the literature recently (Ref 38). The researcher indicated the formation of SiO2 precipitates located just below the external oxide scale.
Su et al. (Ref 39) found that the stainless steel oxidized at 1000 °C in air composition for 3 h (N2-21 vol.% O2) results in the formation of continuous Cr2O3 and SiO2 layers on the alloy and prevents the diffusion of oxygen from the surface to the matrix. This study shows that the exposure of 316L AM SS in the oxidizing atmosphere has a limit in the temperature range of 800-900 °C. The oxidation process at the highest temperature accelerated the formation of rich Fe-based oxide, as shown in Fig. 9(D). A thick oxide product developed with alternative layers enriched in Fe and Cr. However, the outermost layer showed similar concentration of Fe and Cr. The top part of the oxide scale was enriched in Mn, and the bottom was enriched in Si. These findings may have concluded that the outermost layer of the oxide scale was enriched in Mn-Cr spinel and Fe-Cr spinel, whereas the bottom part developed an enriched layer of SiO2.
3.3 Hot Tensile Testing
The true stress versus true strain hot tensile curves of the 316L AM are shown in Fig. 10. Temperature and strain rates impact the stresses. Despite the slightly higher elongation to fracture for the test carried out at 900-0.001, 700-0.01 and 700 °C-0.1 s−1, the other conditions have comparable elongation values. A fast work hardening followed by a plateau due to dynamic recovery is observed for the tests carried out at 800-0.001, 800-0.01, 900-0.001 and 900 °C-0.01 s−1. An initial hardening followed by a second hardening regime up to the peak stress followed by fracture is observed for the tests carried out at 700-0.1 and 800 °C-0.1 s−1.
Dynamic recovery is intense for high-stacking fault energy materials, and low-angle grain boundaries form well-established subgrains (Ref 40, 41). At high temperatures, continuous dynamic recrystallization occurs in high-stacking fault energy materials. The 316L is an FCC material with low stacking fault energy. In this case, dynamic recovery is limited to annihilating or reorganizing the dislocations. A substructure that evolves via a progressive increase in boundary misorientation via continuous dynamic recovery does not occur (Ref 40). For low-stacking fault energy materials, dislocations are pile-ups, and dynamic recrystallization occurs via nucleation and growth (Ref 40, 42), i.e., discontinuous dynamic recrystallization. Dynamic recovery, then, counterbalances the production of dislocations in the early stages of plastic deformation, and it is responsible for the plateau observed in some flow curves in Fig. 11. If discontinuous dynamic recrystallization occurs, it produces un-deformed grains, reducing the dislocation density. Thus, a flow softening can occur. The strain hardening rate evolution of the hot tensile tested 316L AM is shown in Fig. 11. The first stage of work hardening occurs up to a strain of approximately 0.025 for the 316L AM. The second and third regime, when a progressive decrease in strain hardening rate occurs, is only observed for the tests carried out at 700-0.1 and 800 °C-0.1 s−1. The second regime of strain hardening rate decreases from the tests carried out at 700-0.1 and 700 °C-0.01 s−1 up to the test carried out at 800 °C-0.1 s−1. Thus, increasing the temperature from 700 to 800 °C has a higher impact in softening the 316L AM than decreasing the strain rate from 0.1 to 0.01 s−1 at 700 °C.
Table 3 summarizes the values of yield stress (YS), the peak stress and the elongation till fracture of the investigated 316L AM. The yield stress values of the investigated 316L AM range from 85 MPa at 900 °C-0.001 s−1 to 275 MPa at 700 °C-0.001 s−1, as shown in Fig. 12(a). The lower the temperature, the higher the resistance to the dislocation motion, consequently, the higher the yield stress. If the material has a positive strain rate sensitivity, the higher the strain rate, the higher the yield stress. Such behavior occurs at 800 and 900 °C. At 700 °C, though, 0.001 s−1 shows the highest yield stress values.
The maximum stress values (peak stress) range from 95 MPa at 900 °C-0.001 s−1 to 385 MPa at 700 °C-0.1 s−1. The yield stress decreases with a decrease in strain rate at 800 and 900 °C, and its difference is higher at 900 °C. However, a nearly independent strain rate, with a slight tendency of higher yield stress values for low strain rates, is observed at 700 °C. The peak stress is related to the yield stress and to the work hardening that occurs during plastic deformation. Higher temperatures lead to lower yield stress values and promote a more intense dynamic recovery, consequently, smaller peak stress values. Higher values of strain rate produce a more intense work hardening as the time for dynamic recovery is limited. Consequently, the higher the strain rate, the higher the peak stress.
The relative fractions of the difference between maximum stress and yield stress are shown in Fig. 12(b). They are relatively small and in the range of 0.15 to 0.18 for the strain rate of 0.001 s−1, while it increases with an increase in strain rate. It indicates that the role of the restoration mechanisms increases sharply from 700 to 800 °C. At 900 °C, the work hardening is notably reduced compared to 700 °C.
The values of elongation till fracture vary from 10 to 22%, and it is strongly influenced by defects inherent of the L-PBF process. Thus, a tendency with respect to temperature and strain rate cannot be established.
4 Summary and Conclusions
High-temperature oxidation was carried out to investigate the oxide layer formation in dry air. A commercial wrought (CM) 316L and an L-PBF-produced (AM) 316L were used for the oxidation experiments. The microstructure of the oxides was analyzed using scanning electron microscopy. Finally, the L-PBF printed 316L was hot tensile tested to investigate the effect of the temperature and strain rate on the hardening and yield stress behavior. The results allow the following conclusions:
-
The materials show good oxidation resistance up to 700 °C. In addition, both materials developed thick non-protective scales rich in Fe-based oxides at higher temperatures.
-
Increased temperature increases Cr concentration in 316L AM SS up to 40 at.% at 800, and at 900 °C, rich Fe-based oxide formed.
-
Slightly thicker oxide scales were formed in CM than in 316L AM SS material.
-
A fast work hardening occurs for all hot tensile tested samples up to a strain of approximately 0.025. Low temperatures and high strain rates within the investigated range promote a second work hardening regime, while a plateau in the flow stress is observed at high temperatures and low strain rates.
-
The yield stress is nearly independent of the strain rate at 700 °C. It decreases with a decrease in strain rate for 800 and 900 °C, and it decreases with an increase in temperature.
References
P. Samal and J. Newkirk, Powder Metallurgy Stainless Steels, Powder Metall., 2015, 7, p 1–7.
H. Frenz, Guidelines for Methods of Testing and Research in High Temperature Corrosion. Edited by H. J. Grobke and D. B. Meadowcroft, European Federation of Corrosion Publications Number 14, Published by The Institute of Materials, London 1995, ISBN 0-901716-78-2, Mater. Corros., 1997, 48, p 556–557. https://doi.org/10.1002/maco.19970480817
A. Bandyopadhyay, K.D. Traxel, M. Lang, M. Juhasz, N. Eliaz, and S. Bose, Alloy Design via Additive Manufacturing: Advantages, Challenges, Applications and Perspectives, Mater. Today, 2022, 52, p 207–224. https://doi.org/10.1016/j.mattod.2021.11.026
T. Pereira, J.V. Kennedy, and J. Potgieter, A Comparison of Traditional Manufacturing Versus Additive Manufacturing, the Best Method for the Job, Procedia Manuf., 2019, 30, p 11–18. https://doi.org/10.1016/j.promfg.2019.02.003
B.A. Praveen, N. Lokesh, A. Buradi, N. Santhosh, B.L. Praveena, and R. Vignesh, A Comprehensive Review of Emerging Additive Manufacturing (3D Printing Technology): Methods, Materials, Applications, Challenges, Trends and Future Potential, Mater. Today Proc., 2022, 52, p 1309–1313. https://doi.org/10.1016/j.matpr.2021.11.059
H.R. Kotadia, G. Gibbons, A. Das, and P.D. Howes, A Review of Laser Powder Bed Fusion Additive Manufacturing of Aluminium Alloys: Microstructure and Properties, Addit. Manuf., 2021, 46, p 102155. https://doi.org/10.1016/j.addma.2021.102155
N. Li, S. Huang, G. Zhang, R. Qin, W. Liu, H. Xiong, G. Shi, and J. Blackburn, Progress in Additive Manufacturing on New Materials: A Review, J. Mater. Sci. Technol., 2019, 35, p 242–269. https://doi.org/10.1016/j.jmst.2018.09.002
H. Buscail, S. El Messki, F. Riffard, S. Perrier, R. Cueff, E. Caudron, and C. Issartel, Characterisation of the Oxides Formed at 1000°C on the AISI 316L Stainless Steel—Role of Molybdenum, Mater. Chem. Phys., 2008, 111, p 491–496. https://doi.org/10.1016/j.matchemphys.2008.04.054
Q. Jin, J. Li, Y. Xu, X. Xiao, W. Zhang, and L. Jiang, High-Temperature Oxidation of Duplex Stainless Steels S32101 and S32304 in Air and Simulated Industrial Reheating Atmosphere, Corros. Sci., 2010, 52, p 2846–2854. https://doi.org/10.1016/j.corsci.2010.04.033
S. Benafia, D. Retraint, S. Yapi Brou, B. Panicaud, and J.L. Grosseau Poussard, Influence of Surface Mechanical Attrition Treatment on the Oxidation Behaviour of 316L Stainless Steel, Corros. Sci., 2018, 136, p 188–200. https://doi.org/10.1016/j.corsci.2018.03.007
X. Hu, B. Zhang, S. Chen, F. Fang, and J. Jiang, Oxide Scale Growth on High Carbon Steel at High Temperatures, J. Iron Steel Res. Int., 2013, 20, p 47–52. https://doi.org/10.1016/S1006-706X(13)60043-6
A. Bautista, F. Velasco, M. Campos, M.E. Rabanal, and J.M. Torralba, Oxidation Behavior at 900 °C of Austenitic, Ferritic, and Duplex Stainless Steels Manufactured by Powder Metallurgy, Oxid. Met., 2003, 59, p 373–393. https://doi.org/10.1023/A:1023000329514
A.V.C. Sobral, C.V. Franco, M.P. Hierro, F.J. Pérez, and W. Ristow Jr., Oxidation of Injection Molding 316L Stainless Steel at High Temperature, Mater. Corros., 2000, 51, p 791–796. https://doi.org/10.1002/1521-4176(200011)51:11%3c791::AID-MACO791%3e3.0.CO;2-1
V.B. Trindade, U. Krupp, P.E.-G. Wagenhuber, and H.-J. Christ, Oxidation Mechanisms of Cr-Containing Steels and Ni-Base Alloys at High-Temperatures – Part I: The Different Role of Alloy Grain Boundaries, Mater. Corros., 2005, 56, p 785–790. https://doi.org/10.1002/maco.200503879
F. Riffard, H. Buscail, E. Caudron, R. Cueff, C. Issartel, and S. Perrier, Yttrium implantation Effect on 304L Stainless Steel High Temperature Oxidation at 1000 °C, J. Mater. Sci., 2002, 37, p 3925–3933. https://doi.org/10.1023/A:1019667825476
A.M. Huntz, A. Reckmann, C. Haut, C. Sévérac, M. Herbst, F.C.T. Resende, and A.C.S. Sabioni, Oxidation of AISI 304 and AISI 439 Stainless Steels, Mater. Sci. Eng. A, 2007, 447, p 266–276. https://doi.org/10.1016/j.msea.2006.10.022
M.J. Capitán, A. Paúl, J.L. Pastol, and J.A. Odriozola, X-Ray Diffraction Study of Oxide Scales Formed at High Temperatures on AISI 304 Stainless Steel After Cerium Deposition, Oxid. Met., 1999, 52, p 447–462. https://doi.org/10.1023/A:1018812132483
L. Kumar, R. Venkataramani, M. Sundararaman, P. Mukhopadhyay, and S.P. Garg, Studies on the Oxidation Behavior of Inconel 625 Between 873 and 1523 K, Oxid. Met., 1996, 45, p 221–244. https://doi.org/10.1007/BF01046827
IC & E. on P.M.& P. Materials, MPI Federation, Advances in powder metallurgy and particulate materials, 1994. Proceedings of the 1994 International Conference & Exhibition on Powder Metallurgy & Particulate Materials, May 8-11, 1994, Westin Harbour Castle Hotel, Toronto, Canada/Sponsored by the Mettal in: International Conference Exhibition on Powder Metallurgy and Particulate Materials, (Metal Powder Industries Federation, Princeton, NJ, Toronto, 1994). https://www.tib.eu/de/suchen/id/TIBKAT%3A181837595
X. Huang, K. Xiao, X. Fang, Z. Xiong, L. Wei, P. Zhu, and X. Li, Oxidation Behavior of 316L Austenitic Stainless Steel in High Temperature Air with Long-Term Exposure, Mater. Res. Express, 2020, 7, p 066517. https://doi.org/10.1088/2053-1591/ab96fa
W.S.W. Harun, R.I.M. Asri, F.R.M. Romlay, S. Sharif, N.H.M. Jan, and F. Tsumori, Surface Characterisation and Corrosion Behaviour of Oxide Layer for SLMed-316L Stainless Steel, J. Alloys Compd., 2018, 748, p 1044–1052. https://doi.org/10.1016/j.jallcom.2018.03.233
R. Wonneberger, M. Seyring, K. Freiberg, A. Carlsson, J. Rensberg, B. Abendroth, H. Stöcker, M. Rettenmayr, and A. Undisz, Oxidation of Stainless Steel 316L–Oxide Grains with Pronounced Inhomogeneous Composition, Corros. Sci., 2019, 149, p 178–184. https://doi.org/10.1016/j.corsci.2018.12.035
C. Siri, I. Popa, A. Vion, C. Langlade, and S. Chevalier, Impact of Selective Laser Melting Additive Manufacturing on the High Temperature Behavior of AISI 316L Austenitic Stainless Steel, Oxid. Met., 2020, 94, p 527–548. https://doi.org/10.1007/s11085-020-10005-8
N.P. Lavery, J. Cherry, S. Mehmood, H. Davies, B. Girling, E. Sackett, S.G.R. Brown, and J. Sienz, Effects of Hot Isostatic Pressing on the Elastic Modulus and Tensile Properties of 316L Parts Made by Powder Bed Laser Fusion, Mater. Sci. Eng. A, 2017, 693, p 186–213. https://doi.org/10.1016/j.msea.2017.03.100
C. Qiu, M. Al Kindi, A.S. Aladawi, and I. Al Hatmi, A Comprehensive Study on Microstructure and Tensile Behaviour of a Selectively Laser Melted Stainless Steel, Sci. Rep., 2018, 8, p 7785. https://doi.org/10.1038/s41598-018-26136-7
Z. Li, T. Voisin, J.T. McKeown, J. Ye, T. Braun, C. Kamath, W.E. King, and Y.M. Wang, Tensile Properties, Strain Rate Sensitivity, and Activation Volume of Additively Manufactured 316L Stainless Steels, Int. J. Plast., 2019, 120, p 395–410. https://doi.org/10.1016/j.ijplas.2019.05.009
E. Liverani, S. Toschi, L. Ceschini, and A. Fortunato, Effect of Selective Laser Melting (SLM) Process Parameters on Microstructure and Mechanical Properties of 316L Austenitic Stainless Steel, J. Mater. Process. Technol., 2017, 249, p 255–263. https://doi.org/10.1016/j.jmatprotec.2017.05.042
Y.M. Wang, T. Voisin, J.T. McKeown, J. Ye, N.P. Calta, Z. Li, Z. Zeng, Y. Zhang, W. Chen, T.T. Roehling, R.T. Ott, M.K. Santala, P.J. Depond, M.J. Matthews, A.V. Hamza, and T. Zhu, Additively Manufactured Hierarchical Stainless Steels with High Strength and Ductility, Nat. Mater., 2018, 17, p 63–71. https://doi.org/10.1038/nmat5021
L. Liu, Q. Ding, Y. Zhong, J. Zou, J. Wu, Y.-L. Chiu, J. Li, Z. Zhang, Q. Yu, and Z. Shen, Dislocation Network in Additive Manufactured Steel Breaks Strength–Ductility Trade-Off, Mater. Today, 2018, 21, p 354–361. https://doi.org/10.1016/j.mattod.2017.11.004
S. Dryepondt, P. Nandwana, P. Fernandez-Zelaia, and F. List, Microstructure and High Temperature Tensile Properties of 316L Fabricated by Laser Powder-Bed Fusion, Addit. Manuf., 2021, 37, p 101723. https://doi.org/10.1016/j.addma.2020.101723
B. AlMangour, M.-S. Baek, D. Grzesiak, and K.-A. Lee, Strengthening of Stainless Steel by Titanium Carbide Addition and Grain Refinement during Selective Laser Melting, Mater. Sci. Eng. A, 2018, 712, p 812–818. https://doi.org/10.1016/j.msea.2017.11.126
B. AlMangour, Y.-K. Kim, D. Grzesiak, and K.-A. Lee, Novel TiB2-Reinforced 316L Stainless Steel Nanocomposites with Excellent Room- and High-Temperature yield Strength Developed by Additive Manufacturing, Compos. Part B Eng., 2019, 156, p 51–63. https://doi.org/10.1016/j.compositesb.2018.07.050
H.J. Ellingham, Reducibility of Oxides and Sulphides, J. Soc. Chem. Ind., 1944, 63, p 125–160.
J.F. Montoya Carvajal, E.A. Chavarriaga Miranda, S. Villada Gil, A.M. MuñozGarcía, and Ó.J. Restrepo Baena, Síntesis y Caracterización de la Espinela FeCr2O4, Prod. Limpia, 2015, 10, p 64–72.
R. Sachitanand, M. Sattari, J.-E. Svensson, and J. Froitzheim, Evaluation of the Oxidation and Cr Evaporation Properties of Selected FeCr Alloys Used as SOFC Interconnects, Int. J. Hydrog. Energy, 2013, 38, p 15328–15334. https://doi.org/10.1016/j.ijhydene.2013.09.044
G.R. Holcomb and D.E. Alman, The Effect of Manganese Additions on the Reactive Evaporation of Chromium in Ni-Cr Alloys, Scr. Mater., 2006, 54, p 1821–1825. https://doi.org/10.1016/j.scriptamat.2006.01.026
Z.G. Yang, G. Xia, J.W. Stevenson, and P. Singh, Observations on the Oxidation of Mn-Modified Ni-Base Haynes 230 Alloy under SOFC Exposure Conditions, Pacific Northwest National Lab (PNNL), Richland WA, 2005.
W.J. Nowak, Effect of Surface Roughness on Oxidation Resistance of Stainless Steel AISI 316Ti During Exposure at High Temperature, J. Mater. Eng. Perform., 2020, 29, p 8060–8069. https://doi.org/10.1007/s11665-020-05267-x
M. Su, J. Zhao, Z. Tian, C. Gu, and Y. Wang, Improving the High-Temperature Oxidation Resistance of Fe-18Cr-8Ni Austenitic Stainless Steel by Cold-Rolling Treatments, J. Mater. Res. Technol., 2022, 19, p 1510–1524. https://doi.org/10.1016/j.jmrt.2022.05.121
K. Huang and R.E. Logé, A Review of Dynamic Recrystallisation Phenomena in Metallic Materials, Mater. Des., 2016, 111, p 548–574. https://doi.org/10.1016/j.matdes.2016.09.012
F.J. Humphreys and M. Hatherly, Recrystallization and Related Annealing Phenomena, Elsevier, Amsterdam, 2004.
F. Montheillet and J. Le Coze, Influence of Purity on the Dynamic Recrystallisation of Metals and Alloys, Phys. Status Solidi, 2002, 189, p 51–58. https://doi.org/10.1002/1521-396X(200201)189:1%3c51::AID-PSSA51%3e3.0.CO;2-M
Acknowledgments
The KMM-VIN partnership financed the work between Łukasieiwcz—Krakow Institute of Technology and Graz University of Technology. RHB fosters the CD-Laboratory for Design of High-Performance Alloys by Thermomechanical Processing with the support of the Christian Doppler Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dudziak, T., Buzolin, R., Rzad, E. et al. Effect of 316L Stainless Steel Fabrication on Oxidation Resistance, Surface Morphology, and Hot Tensile Behavior. J. of Materi Eng and Perform 32, 10443–10454 (2023). https://doi.org/10.1007/s11665-023-08321-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08321-6