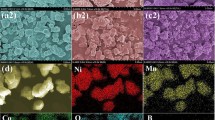
Abstract
Molybdenum trioxide holds the promise of high-performance cathodes for lithium-ion batteries. This study introduces a facile one-step method for large-scale synthesizing B, C co-doped α-MoO3 single crystals by simultaneous decomposition of boric acid, carburization and vapor deposition on a molybdenum substrate. Experimental characterizations indicate that the B and C co-doping promotes the carrier mobility by inducing impurity states near the Fermi level. Additionally, when subjected to the high voltage, the Mo-C and Mo-B bonds become the controlling factors for enhancing the structural stability through the reduced interplanar spacing which facilitates the restriction of transition-metal ion migration. The target material delivers a high discharge capacity of 305 mAh g−1 with initial Coulombic efficiency of 98%, and shows remarkable cycle stability with a specific capacity of 280 mAh g−1 after 80 cycles. This study sheds light on a new strategy for designing and controlling of the doping activity in other transition-metal-oxides for next-generation lithium-ion batteries.






Similar content being viewed by others
References
J.M. Tarascon and M. Armand, Issues and Challenges Facing Rechargeable Lithium Batteries, Nature, 2001, 414(6861), p 359–367.
T. Brezesinski, J. Wang, S.H. Tolbert et al., Ordered Mesoporous Alpha-MoO3 with ISO-Oriented Nanocrystalline Walls for Thin-Film Pseudocapacitors, Nature Mater., 2010, 9(2), p 146–151.
J. Cabana, L. Monconduit, D. Larcher et al., Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions, Adv. Mater., 2010, 22(35), p 170–192.
M.V. Reddy, G.V.S. Rao and B.V.R. Chowdari, Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries, Chem. Rev., 2013, 113(7), p 5364–5457.
Y. Li, H. Sun, X. Cheng et al., In-situ TEM Experiments and First-Principles Studies on the Electrochemical and Mechanical Behaviors of α-MoO 3 in Li-Ion Batteries, Nano Energy, 2016, 27, p 95–102.
Q. Xia, H. Zhao, Z. Du et al., Synthesis and Electrochemical Properties of MoO3/C Composite as Anode Material for Lithium-Ion Batteries, J. Power Sources, 2013, 226(6), p 107–111.
L. Cai, P.M. Rao and X. Zheng, Morphology-Controlled Flame Synthesis of Single, Branched, and Flower-like α-MoO3 Nanobelt Arrays, Nano Lett., 2011, 11(2), p 872–877.
S. Berthumeyrie, J.C. Badot, J.P. Pereiraramos et al., Influence of Lithium Insertion on the Electronic Transport in Electroactive MoO3 Nanobelts and Classical Powders: Morphological and Particle Size Effects, J. Phys. Chem. C, 2010, 114(46), p 19803–19814.
J. Qiu, Z. Yang and Y. Li, N-Doped Carbon Encapsulated Ultrathin MoO3 Nanosheets as Superior Anodes with High Capacity and Excellent Rate Capability for Li-Ion Batteries, J. Mater. Chem. A, 2015, 3(48), p 24245–24253.
S.H. Choi and Y.C. Kang, Crumpled Graphene-Molybdenum Oxide Composite Powders: Preparation and Application in Lithium-Ion Batteries, Chemsuschem, 2014, 7(2), p 523–528.
M.A. Ibrahem, F.Y. Wu, D.A. Mengistie et al., Direct Conversion of Multilayer Molybdenum Trioxide to Nanorods as Multifunctional Electrodes in Lithium-Ion Batteries, Nanoscale, 2014, 6(10), p 5484–5490.
P. Meduri, E. Clark, J.H. Kim et al., MoO3, Nanowire Arrays As Stable and High-Capacity Anodes for Lithium Ion Batteries, Nano Lett., 2012, 12(4), p 1784–1788.
M.F. Hassan, Z.P. Guo, Z. Chen et al., Carbon-Coated MoO 3 Nanobelts as Anode Materials for Lithium-Ion Batteries, J. Power Sources, 2010, 195(8), p 2372–2376.
X. Wei, L. Jiao, S. Liu et al., Synthesis and Electrochemical Performance of V 2 O 5 Doped MoO3 Cathode Materials, J. Alloy. Compd., 2009, 486(1–2), p 672–676.
L. Zhang, G. Xia, Z. Guo et al., In Situ Fabrication of Three-Dimensional Nitrogen and Boron Co-Doped Porous Carbon Nanofibers for High Performance Lithium-Ion Batteries, J. Power Sources, 2016, 324, p 294–301.
D.E. Andreev, Y.S. Vdovin, V.I. Yukhvid et al., Tailoring the Composition and Structure of Nb-, Si-, and B-Doped Mo-Based Composite Materials in the Self-Propagating High-Temperature Synthesis Metallurgy Process, Inorg. Mater., 2020, 56(12), p 1265–1270.
A.M. Hashem, H. Groult, A. Mauger et al., Electrochemical Properties of Nanofibers α-MoO 3 as Cathode Materials for Li Batteries, J. Power Sources, 2012, 219, p 126–132.
O. Kamoun, A. Boukhachem, M. Amlouk et al., Physical Study of Eu Doped MoO3 Thin Films, J. Alloy Compd., 2016, 687, p 595–603.
T. Rauscher, C.I. Müller, A. Schmidt et al., Ni–Mo–B Alloys as Cathode Material for Alkaline Water Electrolysis, Int. J. Hydrog. Energy, 2016, 41(4), p 2165–2176.
J.X. Zhu, D.F. Zhou, S.R. Guo et al., Grain Boundary Conductivity of High Purity Neodymium-Doped Ceria Nanosystem with and Without the Doping of Molybdenum Oxide, J. Power Sources, 2007, 174(1), p 114–123.
N. Zhao, Y. Li, X. Zhao et al., Effect of Particle Size and Purity on the Low Temperature Electrochemical Performance of LiFePO4/C Cathode Material, J. Alloy. Compd., 2016, 683, p 123–132.
R.K. Sharma and G.B. Reddy, Growth of Vertically Aligned α−MoO3 Nanoflakes by Using a Facile PVD Route, AIP Conf. Proc., 2014, 1591(1), p 895–897.
A. Harabor, P. Rotaru, R.I. Scorei et al., Non-conventional Hexagonal Structure for Boric Acid, J. Therm. Anal. Calorim., 2014, 118(2), p 1375–1384.
A.C. Dillon, L.A. Riley, Y.S. Jung et al., HWCVD MoO3 Nanoparticles and aSi for Next Generation Li-Ion Anodes, Thin Solid Films, 2011, 519(14), p 4495–4497.
M. Ullah, E. Ahmed, F. Hussain et al., Electrical Conductivity Enhancement by Boron-Doping in Diamond Using First Principle Calculations, Appl. Surf. Sci., 2015, 334, p 40–44.
Z. Yu, H. Jiang, D. Gu et al., A New Way to Prepare MoO3/C as Anode of Lithium ion Battery for Enhancing the Electrochemical Performance at Room Temperature, J. Electrochem. Sci. Technol., 2016, 7, p 170–178.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Key R&D Program of China (2017YFB0103000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Zhang, H. & Xu, Z. In Situ Formation of Boron and Carbon Co-Doped α-MoO3 Single Crystal as Cathode Materials for High-Performance Lithium-Ion Batteries. J. of Materi Eng and Perform 30, 3484–3491 (2021). https://doi.org/10.1007/s11665-021-05545-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-021-05545-2