Abstract
Ni-Cr and Ti-Si layers were deposited by magnetron sputtering on Ti-46Al-8Nb (at.%) substrates to suppress penetration of gases, which brings about undesirable changes in mechanical properties. Alloy samples with and without surface protection were subjected to interrupted oxidation at elevated temperature (700 and 800 °C) for up to 300 h. Selected mechanical properties as well as adhesion of surface layers were examined in the as-received and oxidized state. Analytical techniques used to characterize surfaces and cross sections of the samples included scanning electron microscopy and light microscopy, energy-dispersive x-ray spectroscopy, x-ray photoelectron spectroscopy and x-ray diffraction. Both investigated layers were sufficiently adherent and effective in hindering gas absorption at 700 °C; however, only Ni-Cr showed promising properties for higher-temperature applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloys based on titanium aluminides are reckoned promising lightweight materials (density of about 4.0 g/cm3) with good mechanical properties and oxidation resistance (Ref 1,2,3,4,5). They might partly replace Ni-superalloys in gas turbine engines (Ref 6), contributing to a 20-30% reduction in total weight and remarkable decrease in fuel consumption. In spite of a number of interesting properties, the application of TiAl alloys is still limited because of their relatively low ductility at room temperature (Ref 7), insufficient oxidation resistance at temperatures exceeding 800 °C (Ref 8) and abrupt embrittlement after short exposure to oxidizing environments at elevated temperatures (Ref 9, 10). Dramatic changes in tensile properties may be brought about by absorption of small-size impurities, such as oxygen, nitrogen, carbon or atoms of inert gases.
A lot of work has been done to enhance oxidation resistance at high temperature and prevent dissolution of gases (Ref 11,12,13). Among these, the most common appeared alloying and surface treatment.
Over the years, various coating compositions and deposition processes were elaborated (Ref 14,15,16,17) to protect titanium aluminides from oxidation and inward diffusion of gases. Among the available techniques, magnetron sputtering is very interesting, because it allows to obtain high-quality surface layers with controllable properties (structure, thickness, etc.) in eco-friendly conditions (Ref 18).
The aim of this study was to apply magnetron-sputtered layers containing nickel and chromium or titanium and silicon on the surface of Ti-46Al-8Nb (at.%) substrates and assess their adhesion, ductility and protective properties. Chemical compositions of layers were selected so as to hamper dissolution of gases in the alloy at moderate temperatures and prevent oxidation at temperatures exceeding 700 °C. The layers based on Ni-Cr and Ti-Si were expected to chemically bond oxygen and convert into relatively thin ceramic layers playing a role of diffusion barriers.
Experimental
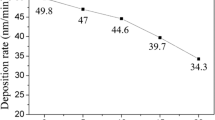
The substrate used in this work, Ti-46Al-8Nb (at.%), was a fully lamellar two-phase alloy: γ-TiAl (75 mol.%) and α2-Ti3Al (25 mol.%). The details of surface preparation and PVD equipment were described in our previous paper (Ref 17). The process parameters are listed in Table 1. The intended thickness of surface layers, about 1-2 µm, was controlled exclusively by varying the deposition time.
Target, 50 mm in diameter and composition Ti-10Si (at.%), was made by hot pressing of elemental powders mixed in suitable proportions. Consolidation of powders required the pressure of 25 MPa and temperature of 1300 °C. The Ni-20Cr (at.%) target was commercially available.
After deposition, the samples were subjected to cyclic oxidation in air at 700 or 800 °C for 80 or 300 h (Ti-Si only). Composition of as-deposited Ti-Si layers was the same as that of the target and slightly different (Ni-27Cr in at.%) for the Ni-Cr layers. The duration of each cycle at high temperature was 1 h (1-h cycles). The exact testing procedure has been described elsewhere (Ref. 17). To avoid losing some oxidation products which might chip off as a result of thermal shocks, the samples were held in alumina crucibles. Mass changes were recorded once a day by weighing samples with and without the crucibles (gross mass and net mass, respectively).
Micromechanical testing was done in agreement with ISO 14577-1, ISO 14577-2, PN-EN ISO 6507-1:2005 on as-coated and oxidized samples, by means of a CSEM Micro-Combi Tester (MCT), with the following fixed parameters: maximum load 10 mN and loading rate 20 mN/min. The properties evaluated in this test comprised maximum penetration depth of the indenter (Hmax), Vickers hardness (HV), indentation hardness (HIT) and Young’s modulus (E). Adhesion of surface layers was determined in a scratch test using Rockwell C diamond intender. The maximum load applied was 30 N (Pmax), and the loading rate was 10 mN/min. Failure phenomena were detected by in situ recording friction, acoustic emission and microscopic examination of the scratch track. Normal forces which brought about first symptoms of failure were referred to as critical loads Lc1 and Lc2, corresponding to the cohesive and adhesive failure, respectively.
Results
Ni-Cr Layers
The as-deposited Ni-Cr layers had smooth surface with a metallic luster. The oxidation behavior of these samples was tested in air at 700 and 800 °C during 80 h. Net mass changes of alloy samples with and without the Ni-Cr layers are presented in Fig. 1. It can be seen that net mass changes of the samples with the Ni-Cr layers are slightly higher than those of bare alloy. Scale spallation was not observed.
Morphology and composition of the scales formed on bare alloy and Ni-Cr layers were obviously different (Fig. 2). The surface of the Ni-Cr layers oxidized at 700 and 800 °C was smooth and covered with small and uniformly distributed crystals, larger than on the bare alloy. Underneath there was a zone with niobium-rich intermetallic precipitates and aluminum oxide embedded in a titanium-rich nitride phase (Ref 21).
Cross sections of the samples with Ni-Cr layers in Fig. 3 reveal an interdiffusion zone under the oxide scale. Thickness of the scale after 80 h of oxidation at 700 and 800 °C is about 2 μm, whereas the interdiffusion zone at 800 °C is more than twice as thick as at 700°C. In the diffusion zone, all alloy components (Ti, Al and Nb) were found accompanied by nickel. The concentration of nickel decreased with the increasing distance from the surface which indicated inward diffusion of this element. Chromium was located mainly in the “oxide” layer. Oxidation of bare Ti-46Al-8Nb (at.%) proceeded differently, as described in our previous papers (Ref 19,20,21). Three layers could be distinguished in the scale formed at 800 °C: very thin outer layer of Al2O3, subjacent layer of TiO2 with some niobium and inner mixed-oxide layer containing all alloy components.
The early stages of oxidation were analyzed by XPS. As can be seen in Table 2, composition of the surface was different for the Ni-Cr layer and bare alloy after the same oxidation time. After 2 h of exposure, the amount of aluminum on alloy surface was twice higher compared with the Ni-Cr layer, whereas the amount of titanium was similar in both cases. In the case of samples with the Ni-Cr layers, the amounts of nickel and aluminum were similar and about twice higher than that of titanium. After 24 h of exposure, the amount of aluminum on the surface increased, indicating an outward diffusion of this element from the underlying alloy. The amount of titanium on the surface of bare alloy decreased after a longer oxidation time and was the same as for the sample with the Ni-Cr layer. The amount of nickel was slightly lower after 24 h of oxidation.
XRD patterns taken from the surface of Ti-46Al-8Nb (at.%) with Ni-Cr layers oxidized at 700 and 800 °C are presented in Fig. 4. Depending on the oxidation temperature, the sample surface had different morphology (Fig. 2) and composition (Fig. 4). The diffractogram of the sample oxidized at 700 °C (Fig. 4) showed only one oxide phase, i.e. Cr2O3, and small amounts of AlNi2Ti and Ni. During oxidation at 800 °C, the following oxides were formed: NiO, NiTiO3, Cr2O3 and TiO2.
Micromechanical properties of samples with the Ni-Cr layers are collected in Table 3. The highest value of penetration depth at the maximum load of 10 mN was found for the unoxidized sample, and it decreased after oxidation. Vickers hardness (HV) and indentation hardness (HIT) were the lowest for the unoxidized layer and increased with the oxidation temperature. The sample oxidized at 800 °C had the highest Young’s modulus.
The results of scratch test are presented in Table 4. The Ni-Cr layers before and after oxidation had good adhesion. The load which caused cohesive cracks inside the scratch track (Lc1) was higher for the oxidized samples. The critical load for adhesive failure (Lc2) exceeded 30 N for the unoxidized layer and was about 26 N for the oxidized ones. However, after oxidation at 800 °C slightly worse adhesion was noted—cracks and delamination were observed close to the scratch track (Fig. 5b).
Ti-Si Layers
Ti-46Al-8Nb (at.%) samples with Ti-Si (at.%) layers were oxidized at 700 or 800 °C for 80 or 300 h. Net mass changes over the oxidation time, shown in Fig. 6, were very similar for bare alloy and alloy with the deposited layer at 700 °C (black lines). At 800 °C the net mass changes of samples with the Ti-Si layer after 80 and 300 h of oxidation were about three times higher compared with the bare alloy.
Surfaces of the alloy samples with and without Ti-Si layers after 80 and 300 h of oxidation at 700 °C were microscopically smooth. In contrast, as illustrated in Fig. 7, at 800 °C the size of crystals growing on the surface of a Ti-Si layer increased with the oxidation time. The oxide layer on the surface of bare alloy remained fine crystalline even after 300 h of exposure.
XRD analyses from the surface of sample oxidized at 800 °C (Fig. 8) revealed TiO2 (rutile) as the predominant phase as well as small amounts of Al2O3 (corundum) and SiO2 (quartz). The large crystals in Fig. 7 are built of rutile. EDS analysis from the surface of sample with the Ti-Si layer oxidized at 800 °C for 300 h showed titanium, aluminum and oxygen only.
Cross sections of samples with a Ti-Si layer after 80 h of oxidation at different temperatures are compared in Fig. 9. At 700 °C, the Ti-Si layer provided good protection to the alloy. The scale was thin and adherent, and there was no evidence of interdiffusion or phase transformations in the subjacent alloy. After the exposure at 800 °C the structure of the near-surface area became more complex. The scale was thick (about 10 µm) and layered. According to EDS, the surface was rich in aluminum and oxygen (Al2O3, confirmed by XRD). The scale was built of titanium, aluminum, silicon and oxygen in different proportions, depending on the location. Close to the scale/alloy interface, there were light-gray and dark-gray precipitates oriented perpendicularly to the substrate (BE image). The lighter precipitates were rich in niobium, whereas the darker ones in aluminum.
Oxidation at 800 °C caused changes in the micromechanical properties of the Ti-Si layer. As shown in Table 5, hardness decreased about three times and the values of Young’s modulus were also lower compared with the unoxidized state. In general, the Ti-Si layers were brittle and prone to delamination. Results of scratch test in Table 6 indicate very low critical loads (Lc1, Lc2) when referred to other layer investigated in this work (Table 4). Cracking and delamination of the Ti-Si layer around the scratch track are clearly visible in Fig. 10.
Discussion
Ni-Cr Layers
The principal idea of using Ni-Cr layers was to protect the Ti-46Al-8Nb (at.%) alloy against gas absorption and oxidation in air. Ni-Cr based coatings are used to improve corrosion and oxidation resistance of steels (Ref 22) and Ti-based alloys (Ref 23). The relatively fast growing non-stoichiometric oxide Ni1−yO with defects in the cation sublattice can hinder an inward diffusion of oxygen. The second oxide, Cr2O3, also defected within the cation sublattice has very low oxygen diffusivity. Doping of nickel (II) oxide with chromium leads to an increased concentration of cation vacancies. For illustration, the NiO layer on a Ni-16.3Cr (at.%) coating contained 0.25-1.5 wt.% chromium (depending on the temperature and oxidation time) which increased the cation vacancy concentration in this layer (Ref 24). The oxidation behavior of Ni-Cr coatings depends on many parameters, such as chromium concentration, deposition method and diffusion phenomena in the coating and the substrate. Thermodynamically Cr2O3 is more stable than NiO but in the real oxidation conditions other factors, e.g., transport properties, may decide about the composition and microstructure of the oxide layer. For illustration, during oxidation of Ni-Cr alloys, containing approximately 10-20 wt.% (11.1-22.0 at.%) chromium, protective Cr2O3 oxide can form, but the concentration of more than about 20 wt.% (22 at.%) of Cr is necessary to secure growth of a continuous Cr2O3 layer (Ref 25). At lower concentrations, 10-20 wt.% Cr, the scale consists of a mixture of Cr2O3, NiO and a NiCr2O4 spinel. NiCr2O4 can be formed when the concentration of chromium in NiO exceeds the solubility limit, which at 850 °C is reached around 1 wt.% Cr or even less. NiCr2O4 can be also produced in a solid state reaction between NiO and Cr2O3 (Ref 25). Compositions of oxide scales on Ni-Cr layers with 20, 40, 60 and 80 at.% Ni were studied in a work devoted to oxidation protection of Ti-6Al-4 V (at.%) at the temperature of 750, 850 or 950 °C (Ref 26). It has been shown that independently of the temperature and coating composition (Cr concentration) the scale was built of two oxides: Cr2O3 and NiO but in different proportions and morphology (continuous single-phase or mixed-oxide layers). The best protection was provided when the concentration of chromium exceeded 60 at.% and a continuous layer of Cr2O3 formed on the surface (Ref 26).
In this work, the net mass changes of Ti-46Al-8Nb (at.%) samples with Ni-Cr layers were slightly larger than for the bare alloy (Fig. 1). It should be underlined, however, that these mass changes were related to the formation of different oxides. According to the XPS (Table 2), Al2O3 and NiO might appear at the early stages of oxidation. Al2O3 was also found in the outermost part of the scale after 300 h of oxidation at 700 and 800 °C.
The cross sections in Fig. 3 indicate that the Ni-Cr layer was perfectly protective. The scale was very thin, compact and adherent. One troublesome effect was relatively rapid inward diffusion of nickel, which might influence mechanical properties of the alloy. As demonstrated in Fig. 3, Ni diffused preferentially along the darker and thicker lamellas. These are apparently built of γ-TiAl, constituting 75 mol.% in the Ti-46Al-8Nb (at.%) alloy (thicker lamellas). In order to prevent the inward diffusion of nickel, an interlayer would be advisable, built of elements having low diffusivity in Ti-Al phases, such as chromium. According to the literature, diffusion of Ni is faster in α2-Ti3Al than in γ-NiAl (Ref 27). Ni diffusion coefficient at 800 °C was measured as 8.0 × 10−15 m2 s−1 in α2-Ti3Al (Ref 28) and 8.0 × 10−19 m2 s−1 in γ-TiAl (Ref 29). On the basis of these data, Ni penetration depth in α2-Ti3Al and γ-TiAl would be 50 and 0.5 μm, respectively, at 800 °C, which by no means are close to the experimental observations. Several plausible explanations can be given to this fact. First of all, the diffusion mechanisms involved may be different. The reported values are the 63Ni isotope diffusion coefficients measured by a radiotracer technique in bulk polycrystalline α2-Ti3Al or γ-TiAl. In this work, the Ti-46Al8Nb (at.%) alloy contained both phases, with γ-TiAl being predominant and the ternary addition of niobium, which is known to affect interdiffusion in Ti-Al phases (Ref 30).The lamellar microstructure of the alloy might influence the transport processes (e.g., favor the interlamellar diffusion). Moreover, nickel diffused into the Ti-Al intermetallic alloy from a Ni-Cr layer, so its chemical potential and diffusivity in this layer were different compared with unalloyed nickel. Finally, in the investigated system, reactive diffusion resulted in the formation of ternary intermetallics, so defect structure and transport properties of these intermediate phases might actually be responsible for the growth rate of the interdiffusion zone. From the diffractogram in Fig. 4, it follows that Ni-Cr layers sufficiently well-prevented oxidation of the Ti-46Al8Nb (at.%) alloy up to 700 °C, because at 800 °C TiO2 was already found in the scale (Fig. 4). At the lower temperature, chromium was oxidized preferentially and a protective Cr2O3 layer was produced on the surface (Fig. 4). Ternary intermetallic, TiNi2Al, formed as a result of nickel diffusion into the alloy. The TiNi2Al (τ4) phase has been identified in Ti-Al-Ni alloys at the temperature of 900 °C (Ref 31, 32). It has been also detected as one of the products of interaction between a Ti-Al-based alloy and a Ni-based alloy (type GH99) after heating from 960 to 1040 °C for 1 to 30 min in a vacuum furnace (1 MPa) (Ref 33). It should be emphasized that some of Ti-Ni and Ni-Al intermetallic phases are known for very good wear and corrosion resistance (Ref 34, 35), so their occurrence in the diffusion zone near the surface might be considered advantageous.
The scale formed at 800 °C was composed of Cr2O3 accompanied by NiO and NiTiO3, indicating some outward diffusion of titanium. On the other hand, TiO2 growth was significantly reduced by the compact layer of Cr2O3.
Ti-Si Layers
Deposition of Ti-Si layers was motivated by the known positive effect of silicon on the oxidation behavior of titanium alloys (Ref 36, 37). Protective properties of silicon-containing coatings have been also reported in other systems (Ref 38, 39). In the case of Ti-Si, it was expected that at the beginning of oxidation silicon would react with oxygen to form partly amorphous SiO2, playing a role of barrier against oxygen penetration along the easy diffusion paths (grain boundaries) in the oxide layer. Unfortunately, experimental results did not confirm these expectations.
It can be concluded from Fig. 9 that at 700 °C the Ti-Si layer can protect the alloy against oxidation in air. The subjacent alloy remained unchanged (no evidence of phase transformations). Interpretation of EDS analysis in Fig. 9 should be careful because the resolution of this method is not good enough for thin (about 2 µm) layers. Moreover, it is difficult to compare concentrations of light and heavy elements as determined by EDS. It can be derived from the EDS analysis, however, that the scale formed at 700 °C contained titanium and silicon oxides. It is interesting to note that some amount of aluminum was also present in the surface layer (point 2, 700 °C), as a result of an outward diffusion of this element from the substrate. The concentration gradient of silicon at the inner interface with the substrate could bring about further inward diffusion of this element, because silicon is known to be the predominant diffuser in the Ti/Si couples (Ref 40).
The temperature of 800 °C appeared too high for the Ti-Si layer to provide good protection to the alloy. Large TiO2 crystals appeared on its surface after 300 h of exposure (Fig. 9). As follows from the diffractogram in Fig. 8, titanium dioxide had the structure of rutile. Its growth on the Ti-Si layer was rather fast but it was slower than on pure titanium (Ref 41). For illustration, mass gain of titanium (purity 99.94%) after 75 h of oxidation at 800 °C was about 14 mg/cm2 whereas that of Ti-46Al-8Nb (at.%) with the Ti-Si layer was about 1 mg/cm2 after the same time. However, the oxidation rate of Ti-46Al-8Nb (at.%) with the Ti-Si layer was still deemed too high. According to thermodynamic calculations (Ref 42), stable SiO2 scales are formed during oxidation of Ti-Si phases, which contain at least 37.5 at.% Si. The cross section in Fig. 9 (800 °C) reveals very complex structure of the near-surface layers. The scale was rather thick (about 10 µm) and composed of two main layers. The inner part (internal oxidation zone) close to the alloy substrate contained aluminum- and niobium-rich precipitates (dark and light spots in SEM BEI in Fig. 9 (800 °C)) similarly as in the case of uncoated alloy. These correspond to aluminum oxide and niobium-rich intermetallic. The outer part of the scale was similar to that formed on the Ti-6Al-1Mn (at.%) alloy after 240 h of oxidation at 800 °C (Ref 43): thicker layers of titanium dioxide were separated by thinner layers of aluminum oxide. The presence of aluminum in the outer part of the scale indicates an outward diffusion of this element from substrate to the Ti-Si layer.
Summary
The magnetron sputtering technique used in this work had many advantages. Compared with the processes based on diffusional transport, the substrate could be maintained at a relatively low temperature, not exceeding about 300 °C. This prevented changes in alloy microstructure and mechanical properties. Two-component targets used in magnetron sputtering enabled very good control of layer composition. The deposited layers were perfectly compact and adherent. In spite of micrometric thickness, they effectively protected the alloy against gas absorption and oxidation at 700 °C. The oxides (NiO and Cr2O3) which formed on the surface of these Ni-Cr layers, with major point defects within the cation sublattice could hinder the inward diffusion of oxygen. The nanocrystalline/amorphous SiO2, which accompanied titanium dioxide on the surface of the Ti-Si layer, significantly reduced the growth of TiO2. At 800 °C the Ni-Cr layers only preserved good oxidation resistance. Some small amounts of TiO2 were, however, detected in the scale (Fig. 4). TiO2 was the predominant phase on the surface of Ti-Si layers (Fig. 8).
The Ni-Cr and Ti-Si layers on a Ti-46Al8Nb (at.%) differed in micromechanical properties. The highest indentation hardness (11.1 GPa) was measured for the Ni-Cr layer after 80 h of oxidation in air at 800 °C (Table 3). The lowest value (2.3 GPa) was found for Ti-Si oxidized in the same conditions (Table 5). In the case of Young’s modulus (E), very low values (good ductility) were calculated for the Ti-Si layer after oxidation at 800 °C (Table 5) and for the Ni-Cr layer after oxidation at 700°C. This can be ascribed to the fine-grained structure of the scale. The resistance to plastic deformation and crack propagation can be evaluated on the basis of the ratios: HIT/E and H 3IT /E2 (Ref 44). The higher the value of HIT/E and H 3IT /E2, the better the wear resistance. The HIT/E and H 3IT /E2 ratios of the investigated samples are collected in the Table 7.
The H/E ratio of the Ti-Si layer before oxidation was higher than that of Ni-Cr, and it decreased to one-half of the original value after oxidation at 800 °C. In the case of Ni-Cr layers, the H/E value was higher for the oxidized samples. Adhesion of the coating is another very important parameter, which could be estimated from the scratch test (Tables 4 and 6). Before oxidation the Ni-Cr layers had better properties compared with Ti-Si, the critical loads for cohesive (Lc1) and adhesive (Lc2) failure were equal to 10.6 N and more than 30 N, respectively (Table 3). It should be mentioned that the critical load for adhesive failure of about 30-40 N is typical for TiN layers deposited on cutting tools (Ref 45). After oxidation at 700 and 800 °C the Lc1 and Lc2 values for the Ni-Cr layers were lower but still relatively high. According to Table 6, the Ti-Si layer had definitely worse properties. The values of friction coefficient collected in Tables 4 and 6 indicate that the surface of Ni-Cr layers was smooth and could have satisfactory anti-wear and anti-friction properties. The friction coefficient of about 0.2 for the Ni-Cr layers (Table 4) was similar to the values reported for the Ni-25Cr coatings deposited on cast iron (Ref 46).
Conclusions
-
1.
The Ni-Cr and Ti-Si layers provided good protection of the Ti-46Al-8Nb (at.%) alloy against gas absorption and oxidation at 700 °C.
-
2.
The Ni-Cr layer effectively prevented gas penetration and significantly limited TiO2 growth at 800 °C owing to the formation of a protective Cr2O3 scale.
-
3.
Relatively fast inward diffusion of nickel to the subjacent alloy, especially at elevated temperatures, is considered some drawback of the proposed protective system based on the oxidation properties of the Ni-Cr alloys. This issue calls for further studies with a focus on mechanical properties and oxidation behavior in a long-term test.
Abbreviations
- µ :
-
Friction coefficient (−)
- E :
-
Young’s modulus (GPa)
- H IT :
-
Indentation hardness (MPa)
- H max :
-
Maximum penetration depth under a load of 10 mN (nm)
- HV:
-
Vickers hardness (HV)
- I :
-
Cathode current intensity (A)
- L c1 :
-
Critical load for cohesive failure (N)
- L c2 :
-
Critical load for adhesive failure (N)
- P :
-
Cathode emission power (W)
- p Ar :
-
Argon pressure (Pa)
- P max :
-
Maximum load for delamination or chipping of the coating (N)
- t :
-
Coating deposition time (s)
- T :
-
Temperature (°C)
References
K. Kothari, R. Radhakrishnan, and N.M. Wereley, Advances in Gamma Titanium Aluminides and Their Manufacturing Techniques, Prog. Aerosp. Sci., 2012, 55, p 1–16
M. Thomas and M.-P. Bacos, Processing and Characterization of TiAl Based Alloys: Towards an Industrial Scale, Onera Aerosp. Lab., 2011, 3, p 1–11
X. Wu, Review of Alloy and Process Development of TiAl Alloys, Intermetallics, 2006, 14, p 1114–1122
M. Blum, G. Jarczyk, H. Scholz, S. Pleier, P. Busse, H.-J. Laudenberg, K. Segtrop, and R. Simon, Prototype Plant for the Economical Mass Production of TiAl-Valves, Mater. Sci. Eng., 2002, A329–331, p 616–620
V. Lupinc, M. Marchionni, G. Onofrio, M. Nazmy, M. Staubli, B. Phillipsen, and K.-H. Rohne, Processing and Properties of γ-TiAl for Turbocharger and Power Plant Applications, Steels and Materials for Power Plants, P. Neumann, D. Allen, and E. Teuckhoff, Ed., Wiley-VCH Verlag GmbH, London, 2006, p 327–331
A. Lasalmonie, Intermetallics: Why Is It So Difficult to Introduce Them in Gas Turbine Engines?, Intermetallics, 2006, 14, p 1123–1129
Z.B. Jiao, J.H. Luan, and C.T. Liu, Strategies for Improving Ductility of Ordered Intermetallics, Prog. Nat. Sci.-Mater., 2016, 26, p 1–12
G. Meier, F. Pettit, and S. Hu, Oxidation Behavior of Titanium Aluminides, J. de Phys. IV Colloq., 1993, 03(C9), p 395–402
X. Wu, A. Huang, D. Hu, and M.H. Loretto, Oxidation-Induced Embrittlement of TiAl Alloys, Intermetallics, 2009, 17, p 540–552
S.L. Draper and D. Isheim, Environmental Embrittlement of a Third Generation g TiAl Alloy, Intermetallics, 2012, 22, p 77–83
G. Schumacher, F. Dettenwanger, M. Schütze, U. Hornauer, E. Richter, E. Wieser, and W. Möller, Microalloying Effects in the Oxidation of TiAl Materials, Intermetallics, 1999, 7, p 1113–1120
R. Pflumm, S. Friedle, and M. Schütze, Oxidation Protection of g-TiAl-Based Alloys: A Review, Intermetallics, 2015, 56, p 1–14
Zhang Dongbo, Song Guanyu, Xue Zhiyong, Jia Long, and Xu Gang, NiAlHf Coating on TiAl Substrate Prepared by EB-PVD, Rare Metal Mat. Eng., 2014, 43(11), p 2657–2662
H. Zhang, X. Peng, and F. Wang, Fabrication of an Oxidation-Resistant β-NiAl Coating on γ-TiAl, Surf. Coat. Technol., 2012, 206, p 2454–2458
A. Ebach-Stahl and M. Fröhlich, Oxidation Study of Pt-Al Based Coatings on γ-TiAl at 950 °C, Surf. Coat. Technol., 2016, 287, p 20–24
J. Cizek, O. Man, P. Roupcova, K. Loke, and I. Dlouhy, Oxidation Performance of Cold Spray Ti-Al Barrier Coated γ-TiAl Intermetallic Substrates, Surf. Coat. Technol., 2015, 268, p 85–89
M. Mitoraj-Królikowska and E. Godlewska, Oxidation Resistance and Micromechanical Properties of a Ti-46Al-8Nb (at.%) Alloy with Cr-Si Magnetron-Sputtered Coatings, Surf. Coat. Technol., 2018, 350, p 732–739
S.L. Lee, M. Cipollo, F. Yee, R. Wei, K. Coulter, J. Lin, W. Sproul, J.J. Moore, Deposition of Environmental Friendly Tantalum and Chromium Nitride Coatings Using HIPIMS-MPP-PEMS Technologies, 53rd Annual Technical Conference Proceedings, 2010, p 125–133
M. Mitoraj, E. Godlewska, O. Heintz, N. Geoffroy, S. Fontana, and S. Chevalier, Scale Composition and Oxidation Mechanism of the Ti-46Al-8Nb Alloy in Air at 700 and 800 °C, Intermetallics, 2011, 19, p 39–47
E. Godlewska, M. Mitoraj, and J. Morgiel, Reaction and Diffusion Phenomena Upon Oxidation of a (γ+α2) TiAlNb Alloy in Air, Mater. High Temp., 2009, 26(1), p 99–103
E. Godlewska, M. Mitoraj, F. Devred, and B.E. Nieuwenhuys, Reactivity of 459Al-8Nb Alloy in Air at 700-900°C, J. Therm. Anal. Calorim., 2007, 88(1), p 225–230
N.F. Ak, C. Tekmen, I. Ozdemir, H.S. Soykan, and E. Celik, NiCr Coatings on Stainless Steel by HVOF Technique, Surf. Coat. Technol., 2003, 174–175, p 1070–1073
I. Cvijović, M.T. Jovanović, and D. Peruško, Cyclic Oxidation Behaviour of Ti3Al-Based Alloy with Ni-Cr Protective Layer, Corros. Sci., 2008, 50, p 1919–1925
G.C. Wood and T. Hodgkiess, Characteristic Scales on Pure Nickel-Chromium Alloys at 800-1200°C, J. Electrochem. Soc., 1966, 113(4), p 319–327
H.V. Atkinson, A Review of the Role of Short-Circuit Diffusion in the Oxidation of Nickel, Chromium, and Nickel-Chromium Alloys, Oxid. Met., 1985, 24(3/4), p 177–197
Dong-Bo Wei, Ping-Ze Zhang, Zheng-Jun Yao, Xiang-Fei Wei, Jin-Tang Zhou, and Xiao-Hu Chen, Preparation and High-Temperature Oxidation Behavior Of Plasma Cr-Ni Alloying on Ti6Al4 V Alloy Based on Double Glow Plasma Surface Metallurgy Technology, Appl. Surf. Sci., 2016, 388, p 571–578
C. Herzig, T. Przeorski, M. Friesel, F. Hisker, and S. Divinski, Tracer Solute Diffusion of Nb, Zr, Cr, Fe, and Ni in g-TiAl: Effect of Preferential Site Occupation, Intermetallics, 2001, 9, p 461–472
J. Breuer, T. Wilger, M. Friesel, and C. Herzig, Interstitial and Substitutional Diffusion of Metallic Solutes in Ti3Al, Intermetallics, 1999, 7, p 381–388
T. Przeorski, M. Friesel, F. Hisker, S.V. Divinski, C. Herzig, Solute Diffusion in the Intermetallic Compound γ-TiAl, Deffect and Diffusion Forum, 2001, 194–199, p 493–498
S. Divinski, F. Hisker, C. Klinkenberg, and C. Herzig, Niobium and Titanium Diffusion in the High Niobium-Containing Ti-54Al-10Nb Alloy, Intermetallics, 2006, 14, p 792–799
B. Huneau, P. Rogl, K. Zeng, R. Schmid-Fetzer, M. Bohn, and J. Bauer, The Ternary System Al-Ni-Ti Part I: Isothermal Section at 900 °C; Experimental Investigation and Thermodynamic Calculation, Intermetallics, 1999, 7, p 1337–1345
A.U. Khan, X. Yan, P. Rogl, and A. Saccone, On the Four-Phase Reactions in the Ti-Ni-Al System, Intermetallics, 2009, 17, p 1000–1006
Li Hai-xin, He Peng, Lin Tie-song, Pan Feng, Feng Ji-cai, and Huang Yu-dong, Microstructure and Shear Strength of Reactive Brazing Joints of TiAl/Ni-Based Alloy, Trans. Nonferrous Met. Soc. China, 2012, 22, p 324–329
Q.S. Lin, K.S. Zhou, C.M. Deng, M. Liu, L.P. Xu, and C.G. Deng, Deposition Mechanisms and Oxidation Behaviors of Ti-Ni Coatings Deposited in Low-Temperature HVOF Spraying Process, J. Therm. Spray. Technol., 2014, 26(3), p 892–902
Y.I. Evdokimenko, V.M. Kisel, S.V. Buchakov, A.A. Rogozinskaya, D.Z. Yurchenko, and R.V. Litvin, Properties of Intermetallic Ni-Al Coatings Deposited by High-Velocity Air-Fuel Spraying, Powder Metall. Metal., 2011, 49(11-12), p 660–666
A.M. Chaze and C. Coddet, Influence of Silicon on the Oxidation of Titanium Between 550 and 700 °C, Oxid. Met., 1987, 27(1-2), p 1–20
D. Vojtĕch, H. Čížová, K. Jurek, and J. Maixner, Influence of Silicon on High-Temperature Cyclic Oxidation Behaviour of Titanium, J. Alloy. Compd., 2005, 394, p 240–249
H.-P. Xiong, W. Mao, Y.-H. Xie, Y.-Y. Cheng, and X.-H. Li, Formation of Silicide Coatings on the Surface of a TiAl-Based Alloy and Improvement in Oxidation Resistance, Mater. Sci. Eng.: A, 2005, 391, p 10–18
X.Y. Li, S. Taniguchi, Y. Matsunaga, K. Nakagawa, and K. Fujita, Influence of Siliconizing on the Oxidation Behavior of a g-TiAl Based Alloy, Intermetallics, 2003, 11, p 143–150
J. Chang, G.B. Kim, D.-S. Yoon, H.K. Baik, D.-J. Yoo, and S.-M. Lee, Investigation of the Mechanism of Titanium Silicide Reaction Using Ion-Beam-Assisted Deposition, Appl. Phys. Lett., 1999, 75(19), p 2900–2902
P. Kofstad, P.B. Anderson, and O.J. Krudtaa, Oxidation of Titanium in the Temperature Range 800-1200 °C, J. Less-Common Met., 1961, 3, p 89–97
B.V. Cockeram and R.A. Rapp, The Kinetics of Multilayered Titanium-Silicide Coatings Grown by the Pack Cementation Method, Metall. Mater. Trans. A, 1996, 26(4), p 777–791
E. Godlewska, M. Mitoraj, and B. Jajko, Oxidation of Some Titanium Alloys in Air at Elevated Temperatures, Mater. Sci. Forum, 2008, 595-598, p 967–974
Wangyang Ni, Yang-Tse Cheng, Michael J. Lukitsch, Anita M. Weiner, Lenoid C. Lev, and David S. Grummon, Effects of the Ratio of Hardness to Young’s Modulus on the Friction and Wear Behaviour of Bilayer Coatings, Appl. Phys. Lett., 2004, 85, p 4028–4030
J. Valli, U. Mäkelä, A. Matthews, and V. Murawa, TiN Coating Adhesion Studies Using the Scratch Test Method, J. Vac. Sci. Technol., 1985, A3, p 2411–2414
Z. Minlin, L. Wenjin, and Z. Hongjun, Corrosion and Wear Resistance Characteristics of NiCr Coating by Laser Alloying with Powder Feeding on Grey Iron Liner, Wear, 2006, 260, p 1349–1355
Acknowledgment
The authors gratefully acknowledge financial support from AGH-UST statutory funds (badania statutowe: 11.11.160.438) and as well as valuable contributions of Dr. S. Zimowski, Dr. M. Kot and Dr. R. Mania to the measurements of micromechanical properties and coating deposition.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mitoraj-Królikowska, M., Godlewska, E. Magnetron-Sputtered Ni-Cr and Ti-Si Layers to Protect Ti-46Al-8Nb (at.%) Substrates Against Gas Absorption. J. of Materi Eng and Perform 28, 6258–6267 (2019). https://doi.org/10.1007/s11665-019-04327-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-019-04327-1