Abstract
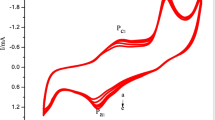
Automobile and transportation on land and in air are the major causes of global environment pollution through the chemical combustion of petroleum fuels. The need of the hour is to run the transportation units by fuel cells which convert fuels to energy through electrochemical oxidation, straight to pure electrical energy with no polluting substances. Development of fuel cell lies on exhaustive research on development of high energetic electrocatalytic electrodes over which the fuels break to produce free electron and ions, delivering electrical energy. The present work aims at developing two electrocatalytic materials: NiO-nanocarbon and MnO2-nanocarbon by electrosynthesis method. The performance of the materials was investigated by electrochemical characterization: cyclic voltammetry, chronoammetry, polarization tests and impedance spectroscopy. The materials were found to deliver a high current on electro-oxidation of glucose. The effect of nanocarbon on NiO and MnO2 has enhanced the current to a level of over 100 mA/cm2. XRD study showed the presence of metal oxides and carbon. The electron microscopy images revealed fine grain oxides with entrapped nanocarbon. The carbon seems to be in the form of graphene, produced during electrochemical exfoliation at anode. The SEM morphology of enhanced 3D surface area with high energetic nanocarbon phase entrapped with fine structure metal oxides accounts for the high current obtained by electro-oxidation of the fuel. The materials developed are found to be very good energetic electrodes for future fuel cell in automotive industry.














Similar content being viewed by others
References
S. Paul, Characterization of Bioelectrochemical Fuel Cell Fabricated with Agriculture Wastes and Surface Modified Electrode Materials, J. Fuel Cell Sci. Technol. ASME, 2012, 9(2), p 021013
S. Paul and A. Jana, Study on Bioelectrochemical Fuel Cell With Algae, J. Inst. Eng. Interdiscip. Div., 2007, 88, p 27–30
S. Paul and P. Mondal, Pyrolysis of Forest Residue for Production of Bio Fuel, Int. Energy J., 2006, 7, p 221–225
S. Paul and P. Mondal, Fabrication and Characterization of Bioelectrochemical Fuel Cell with Pyrolysed Produced Bio Oil and Hydrolysed Biomass by Fermentation, J. Inst. Eng. Interdiscip., 2009, 90, p 40–45
S. Paul and A. Ghosh, Electrochemical Characterization of MnO2 as Electrocatalytic Energy Material for Fuel Cell Electrode, J. Fuel Chem. Technol. Sci. Direct, 2015, 43(03), p 344–351
S. Pau, l Ruma Chatterjee Development of Nano Carbon-MnO2 Energy Material for Glucose Fuel Cell Electrode, Namomater. Energy ICE Publ., 2015, 3, p 1–9
S. Paul and A. Ghosh, MnO2, a High Electrocatalytic Energy Material to Synthesis Energy from Oxidation of Methanol in Fuel Cell, Energy Environ. Focus, 2015, 5, p 1–7
S. Guchhait and S. Paul, Synthesis and Characterization of ZnO-Al2O3 Oxides as Energetic Electrocatalytic Material for Glucose Fuel Cell, J. Fuel Chem. Technol., 2015, 43(08), p 1004–1010
S. Paul, S.K. Naimuddin, and A. Ghosh, Electrochemical Characterization of Ni-Co and Ni-Co-Fe for Oxidation of Methyl Alcohol Fuel with High Energetic Catalytic Surface, J. Fuel Chem. Technol., 2014, 42, p 87–95
S. Paul and S.K. Naimuddin, Electrochemical Characterization of Synthesized Ni-Co and Ni-Co-Fe Electrodes for Methanol Fuel Cell, J. Fuel Cell. Sci. Technol. ASME, 2014, 11, p 18–25
S. Paul, Materials and Electrochemistry: Present and Future Battery, J. Electrochem. Sci. Technol., 2016, 7(2), p 1–17
S. Paul, Electrochemical Energy Synthesis and Storage in Battery and Fuel Cell. Amazon Pub, 2016, pp. 82–112. https://www.amazon.com/dp/1520321929
E. Turkusic, K. Kalcher, K. Schachl, A. Komersova, M. Bartos, H. Moderegger, I. Svancara, and K. Vytras, Amperometric Determination of Glucose with an MnO2 and Glucose Oxidase Bulk-Modified Screen-Printed Carbon Ink Biosensor, Anal. Lett., 2001, 34, p 2633–2647
J.-J. Xu, J.-J. Feng, X. Zhong, and H.-Y. Chen, Low-Potential Detection of Glucose with a Biosensor Based on the Immobilization of Glucose Oxidase on Polymer/Manganese Oxide Layered Nanocomposite, Electroanalysis, 2008, 20, p 507–512
J.-J. Xu, X.-L. Luo, Y. Du, and H.-Y. Chen, Application of MnO2 Nanoparticles as an Eliminator of Ascorbate Interference to Amperometric Glucose Biosensors, Electrochem. Commun., 2004, 6, p 1169–1173
M. Mohamedi, Y. Hisamitsu, K. Kihara, T. Kudo, T. Itoh, and I. Uchida, Ni-Al Alloy as Alternative Cathode for Molten Carbonate Fuel Cells, Orig. Res. Artic. J. Alloys Compd., 2001, 315, p 224–233
K. Suresh Kumar, P. Haridoss, and S.K. Seshadri, Synthesis and Characterization of Electrodeposited Ni-Pd Alloy Electrodes for Methanol Oxidation, Origin. Res. Article. Surf. Coat. Technol., 2008, 202, p 1764–1770
M. Pasta, R. Ruffo, E. Falletta, C.M. Mari, and C. Della Pina, Alkaline Glucose Oxidation on Structured Gold Electrodes, Gold Bull., 2010, 43, p 57–64
J. Chen, H. Zheng, J. Kang, F. Yang, Ya Cao, and M. Xiang, An Alkaline Direct Oxidation Glucose Fuel Cell Using Three-Dimensional Structural Au/Ni-Foam as Catalytic Electrodes, R. Soc. Chem., 2017, 7, p 3035–3042
S. Jukka-Pekka, K. Petri, K. Tanja, S. Jorma, K. Yohannes, S. Kari, and L. Martti, Towards an Efficient Direct Glucose Anion Exchange Membrane Fuel Cell System with Several Electro-Oxidation Units, Int. J. Electrochem. Sci., 2017, 12, p 3697–3708
H. Guo, H. Yin, X. Yan, S. Shi, Q. Yu, Z. Cao, and J. Li, Pt-Bi Decorated Nanoporous Gold for High Performance Direct Glucose Fuel Cell, Sci. Rep., 2016, 6, p 39162
P. Mahanandia, F. Simon, G. Heinrich, and K.K. Nanda, An Electrochemical Method for the Synthesis of Few Layer Graphene Sheets for High Temperature, Applications, Chem. Commun., 2014, 50, p 4613–4615
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paul, S., Paria, A. Development of NiO-Nanocarbon and MnO2-Nanocarbon Energetic Catalytic Electrode Materials to Synthesize Electrical Energy through Electrochemical Oxidation of Glucose. J. of Materi Eng and Perform 28, 4574–4581 (2019). https://doi.org/10.1007/s11665-019-04138-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-019-04138-4