Abstract
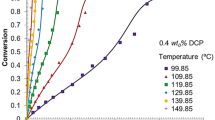
A comprehensive mathematical model and experimental study of single particle growth for styrene polymerization over a silica-supported metallocene catalyst were investigated. The model was developed based on the modification of the well-known multigrain model (MGM) by introducing mesoparticle scale limitations. Thereafter, the model was employed to predict the effects of bulk phase temperature and catalyst properties (initial catalyst active site concentration and initial catalyst particle size) on the polymerization rate, degree of polymerization (DP), and the polydispersity index (PDI) of syndiotactic polystyrene (SPS). The simulation results showed a significant radial distribution of styrene concentration across polymer particle growth at different polymerization conditions. It was found that increasing the initial catalyst concentration and bulk phase temperature resulted in polymerization rate enhancement. In context, the polymerization rate decreased as the initial catalyst particle size increased from 20 to 50 μm. The results revealed that a uniform increase in DP of the polymer was obtained by increasing the initial catalyst concentration and the reaction temperature, while resulting in a decrease of the PDI value. Meanwhile, the DP and PDI values varied inversely under the influence of initial catalyst particle size within a period of time similar to the one needed in the catalyst decay. The simulated results in the study agree well with experimental data of SPS.















Similar content being viewed by others
Abbreviations
- D ef,M,i :
-
Effective macroparticle diffusivity, at the ith grid point (cm2/min)
- D ef,s :
-
Effective mesoparticle diffusivity (cm2/min)
- D ef,μ :
-
Effective microparticle diffusivity (cm2/min)
- D m,solv :
-
Monomer diffusivity in the solvent (cm2/min)
- k p :
-
Propagation rate constant (L/(mol h))
- k d :
-
Catalyst deactivation rate constant (h−1)
- k s :
-
Liquid film mass transfer coefficient (m2/s)
- [M] i :
-
Monomer concentration in the macroparticle, at the ith grid point (mol/L)
- [M]s :
-
Monomer concentration in the mesoparticle (mol/L)
- [M]μ :
-
Monomer concentration in the microparticle (mol/L)
- [M]c :
-
Monomer concentration at the surface of catalyst fragment (mol/L)
- [M]b :
-
Bulk monomer concentration (mol/L)
- N :
-
Number of shell
- r :
-
Radial position at the macroparticle level (m)
- R c :
-
Radius of catalyst subparticles (m)
- R N+2 :
-
Macroparticle radius (m)
- R o :
-
Initial catalyst particle radius (m)
- R h,i :
-
Radius of ith hypothetical shells
- R p,i :
-
Rate of reaction per unit volume at the ith grid point (mol/(m3 s))
- R overall :
-
Overall time-dependent polymerization rate (g SPS/(g cat h))
- \( {{\upeta}}_{\text{s}} \) :
-
Mesoparticle diffusion effectiveness factor
- \( {{\upeta}}_{{{\upmu}}} \) :
-
Microparticle diffusion effectiveness factor
- λLk :
-
kth Moment of live polymers
- λDk :
-
kth Moment of dead polymers
References
M. Malanga, O. Isogai, T. Yamada, S. Iwasaki, and M. Kuramoto, Ed., Historical Overview and Commercialization of Syndiotactic Polystyrene, Syndiotactic Polystyrene, John Wiley & Sons, Inc., New York, 2009, p 1–13. doi:10.1002/9780470557006.ch1
N. Ishihara, T. Seimiya, M. Kuramoto, and M. Uoi, Crystalline Syndiotactic Polystyrene, Macromolecules, 1986, 19(9), p 2464–2465. doi:10.1021/ma00163a027
J.J. Han, H.W. Lee, W.J. Yoon, and K.Y. Choi, Rate and Molecular Weight Distribution Modeling of Syndiospecific Styrene Polymerization Over Silica-Supported Metallocene Catalyst, Polymer, 2007, 48(22), p 6519–6531
J.J. Han, W.J. Yoon, H.W. Lee, and K.Y. Choi, Nascent Morphology of Syndiotactic Polystyrene Synthesized Over Silica-Supported Metallocene Catalyst, Polymer, 2008, 49(19), p 4141–4149
H.W. Lee, J.S. Chung, and K.Y. Choi, Physical Transitions and Nascent Morphology of Syndiotactic Polystyrene in Slurry Polymerization with Embedded Cp*Ti(OMe)3/Methyl Aluminoxane Catalyst, Polymer, 2005, 46(14), p 5032–5039
S. Rahmani, R. Mohammadi, and A.A. Entezami, Comparison of Syndiotactic Polystyrene Morphology Obtained Via Heterogeneous and Homogeneous Polymerization with Metallocene Catalyst, Macromol. Symp., 2008, 274(1), p 43–48. doi:10.1002/masy.200851407
W.R. Schmeal and J.R. Street, Polymerization in Expanding Catalyst Particles, AIChE J., 1971, 17(5), p 1188–1197. doi:10.1002/aic.690170526
W.R. Schmeal and J.R. Street, Polymerization in Catalyst Particles: Calculation of Molecular Weight Distribution, J. Polym. Sci.: Polym. Phys. Ed., 1972, 10(11), p 2173–2187. doi:10.1002/pol.1972.180101106
E.J. Nagel, V.A. Kirillov, and W.H. Ray, Prediction of Molecular Weight Distributions for High-Density Polyolefins, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19(3), p 372–379. doi:10.1021/i360075a016
D. Singh and R.P. Merrill, Molecular Weight Distribution of Polyethylene Produced by Ziegler-Natta Catalysts, Macromolecules, 1971, 4(5), p 599–604. doi:10.1021/ma60023a017
R. Galvan and M. Tirrell, Orthogonal Collocation Applied to Analysis of Heterogeneous Ziegler-Natta Polymerization, Comput. Chem. Eng., 1986, 10(1), p 77–85
R. Galvan and M. Tirrell, Molecular Weight Distribution Predictions for Heterogeneous Ziegler-Natta Polymerization Using a Two-Site Model, Chem. Eng. Sci., 1986, 41(9), p 2385–2393
S. Floyd, K.Y. Choi, T.W. Taylor, and W.H. Ray, Polymerization of Olefins Through Heterogeneous Catalysis. III. Polymer Particle Modelling with an Analysis of Intraparticle Heat and Mass Transfer Effects, J. Appl. Polym. Sci., 1986, 32(1), p 2935–2960. doi:10.1002/app.1986.070320108
P. Varshney, D. Kunzru, and S.K. Gupta, A Multigrain Catalyst Model for Unifunctional Multicomponent Catalysts, Chem. Eng. Res. Des., 2010, 88, p 455–464
J.A. Debling and W.H. Ray, Heat and Mass Transfer Effects in Multistage Polymerization Processes: Impact Polypropylene, Ind. Eng. Chem. Res., 1995, 34(10), p 3466–3480. doi:10.1021/ie00037a035
P. Kittilsen and H.F. Svendsen, Three-Level Mass-Transfer Model for the Heterogeneous Polymerization of Olefins, J. Appl. Polym. Sci., 2004, 91(4), p 2158–2167. doi:10.1002/app.13338
S. Knoke, D. Ferrari, B. Tesche, and G. Fink, Microkinetic Videomicroscopic Analysis of Olefin Polymerization with a Supported Metallocene Catalyst, Angew. Chem. Int. Ed., 2003, 42(41), p 5090–5093. doi:10.1002/anie.200351582
F. Bonini, V. Fraaije, and G. Fink, Propylene Polymerization Through Supported Metallocene/MAO Catalysts: Kinetic Analysis and Modelling, J. Polym. Sci., Part A: Polym. Chem., 1995, 33(14), p 2393–2402. doi:10.1002/pola.1995.080331412
A. Alexiadis, C. Andes, D. Ferrari, F. Korber, K. Hauschild, M. Bochmann, and G. Fink, Mathematical Modeling of Homopolymerization on Supported Metallocene Catalysts, Macromol. Mater. Eng., 2004, 289(5), p 457–466. doi:10.1002/mame.200400011
S.R. Sultan, W.J.N. Fernando, and A.S. Sata, Multiscale Modeling of Syndiospecific Styrene Polymerization, J. Polym. Res., 2012, 19, p 9778
B.A. Finlayson, Nonlinear Analysis in Chemical Engineering, McGraw-Hill International Book Co., New York, 1980
T.K. Sherwood, R.L. Pigford, and C.R. Wilke, Mass Transfer, McGraw-Hill, Kogakusha, Tokyo, 1975
R.C. Reid, J.M. Prausnitz, and B.E. Poling, The Properties of Gases and Liquids, McGraw-Hill, New York, 1987
P. Sarkar and S.K. Gupta, Simulation of Propylene Polymerization: An Efficient Algorithm, Polymer, 1992, 33(7), p 1477–1485
W.E. Ranz and W.R. Marshall, Jr., Evaporation from Drops. Part I, Chem. Eng. Prog., 1952, 48, p 141–146
E.L. Cussler, Diffusion: Mass Transfer in Fluid Systems, Cambridge University Press, Cambridge, 2009
P. Sarkar and S.K. Gupta, Modelling of Propylene Polymerization in an Isothermal Slurry Reactor, Polymer, 1991, 32(15), p 2842–2852
Acknowledgments
The authors would like to thank University Sains Malaysia (USM) for funding this project under Research University Scheme No. (1001/PJKIMIA/811107). The first author gratefully acknowledges the USM for supporting this work under USM Fellowship.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The changes in the shells volume, (∆V i ) and the location of the grid points (R i ) with time are given in this section. As shown in Fig. 2. The hypothetical shell can be defined as (R h, i−1 ≤ r ≤ R h,i ) such that the entire polymer produced (since time t = 0) by the catalyst particles of radius (R c) are accommodated in it. In the interval (t to t + ∆t), the volume of microparticles (V μ,i) in the first shell are given by:
With V μ,i (t = 0) being the initial volume of microparticle in the first shell.
The total volume of polymer (V i ), the volume of mesoparticle (V s,i ) produced at ith shell are given by:
With V i (t = 0) and V s,i (t = 0) being the initial total volume and volume of every polymer mesoparticle of ith volume, respectively.
We can now define the hypothetical shells at any time by:
where \( R_{{{\text{h}},{\text{o}}}} = 0 \)
The radius of meso and microparticle at ith shell being:
The catalyst particles are assumed to be placed at the mid points of each hypothetical shell. Thus:
Then the computational grid points are related to (R 1,i ) by:
The values of (∆r i ) to be used in the equation of Table 2 are given by:
Rights and permissions
About this article
Cite this article
Sultan, S.R., Fernando, W.J.N. & Sata, S.A. Modeling and Experimental Evaluation of Single Particle Growth in Syndiotactic Polymerization of Styrene. J. of Materi Eng and Perform 22, 2148–2160 (2013). https://doi.org/10.1007/s11665-013-0506-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-013-0506-2