Abstract
We conducted a simple solution-based method to fabricate Bi2S3 nanowire thin film solar cells by spin coating with varying sulfur-to-bismuth ratios. Spherical nanoparticles were observed in the thin film with low-concentration sulfur solution, with these nanoparticles gradually changing to nanorods. Finally, nanowires of Bi2S3 were observed in the thin film with a high sulfur concentration in solution. The band gap gradually decreased with the increase in sulfur concentration. The solar cell performance was significantly improved with the nanowire structure. During film fabrication, sulfur vacancy defects appeared primarily because of high annealing temperatures. These defects were somewhat reduced by the high concentration of sulfur in the solution, supported by the energy-dispersive x-ray spectroscopy (EDS) results. The elemental chemical composition of Bi2S3 material showed an increase in the sulfur-to-bismuth ratio, reaching saturation at almost 0.9. In this work, we systematically observed the effect on the optical properties, surface morphology, and photovoltaic properties by changing the concentration of sulfur in the precursor. The nanowire structure with a high concentration of sulfur in the solution is a promising way to improve the Bi2S3 thin film solar cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New materials, technologies, and synthesis methods are constantly being sought to optimize solar cell performance. In recent decades, many intensive studies have been conducted to develop the most efficient perovskite solar cells, and similar work is ongoing in geometric progression. Experimental power conversion efficiency (PCE) of greater than 25%1 has been achieved, but low stability and high toxicity are problems that need to be solved. Today’s research is focused mainly on nontoxic, earth-abundant materials and economical synthesis methods to obtain more efficient solar cells.
Metal chalcogenides such as Bi2S3, Sb2S3, and As2Se3 (type A2B3; A = Bi, Sb, As; B = S, Se, Te) have been studied with great interest due to their appropriate band gap and unique electronic and optical properties. Among these metal chalcogenides, Sb2S3 with band gap energy of 1.7–1.8 eV has been actively studied because of its high photosensitivity and nontoxic nature, with PCE greater than 8% reported recently.2 However, the band gap energy is larger than the optimum value (1.4–1.5 eV), which may be a reason for the difficulty in achieving higher efficiency. Therefore, Bi2S3 has garnered increasing attention because of its suitable band gap between 1.3 eV and 1.7 eV and larger absorption coefficient compared to Sb2S3. However, Bi2S3 has not been studied much for solar cell materials compared with Sb2S3.3,4,5,6,7 Bi2S3 material can be in the form of either molecular crystals or polymeric and layered crystals. Bi2S3 normally shows n-type conductivity,8,9 and in some conditions it also possesses p-type conductivity,3 depending on the deposition temperature and method.10,11 These two types of conductivity offer a wide range of doping possibilities to modify their conductivity and other properties. The low band gap and good conductivity of these nanoparticles have made them attractive materials for various applications. Bi2S3 can exist in various nanostructures including nanostars, nanoballs, nanoflakes, nanorods, nanoflowers, nanobelts, nanotubes, and nanowires. The structure of nanoparticles and their applications depend on the method of synthesis, the nature of the precursors, and the temperature in the synthesis process.9,12
There are various methods for synthesizing Bi2S3 material, and all methods have their advantages and disadvantages.9 Noble or modified methods are always needed for the best outcomes of Bi2S3 material. Papers have reported on the fabrication of Bi2S3 thin film solar cells by chemical deposition,12 rapid thermal evaporation,13 and close-spaced sublimation,3 but with low PCE yield. To date, the highest PCE reported for Bi2S3 thin film solar cells is 0.75%.13 Apart from Bi2S3 thin film solar cells, Bi2S3 quantum dot-sensitized solar cells have a PCE of 3.1%,14 and the cross-linked heterostructure of Bi2S3/TiO2 has the highest efficiency of 3.29%.15 This result highlights the superiority of nanostructures over thin films for improving PCE. In this situation, the use of nanowires will be the future direction to improve Bi2S3 solar cells. Among the many synthesis methods, spin coating is a simple approach for the fabrication of thin film, and this method is also used to fabricate organic solar cells and perovskite solar cells. There are few reports on the characteristics of nanowire Bi2S3 synthesized using the spin coating technique. In this paper, we present the synthesis and characteristics of Bi2S3 nanowire thin film in a solar cell application by spin coating. Nishikubo et al. reported that the low PCE of Bi2S3 thin film solar cells is due to the trade-off between the electronic properties and the morphology.16 However, there is a possibility of overcoming this issue by using nanowire structures because of their high conductivity.
Here, we propose using Bi2S3 nanowire for the solar cell active layer to improve PCE. One-dimensional nanowires have unique optical and electronic properties due to their large surface area and high conductivity. Several papers have reported on the high photoconductivity and improved photodiode properties using Bi2S3 nanowires.17,18 However, there have been no reports comparing Bi2S3 nanowire solar cell properties with Bi2S3 thin film. The current work investigates the fabrication of a Bi2S3 nanowire thin film solar cell on a fluorine-doped tin oxide (FTO) substrate with varying sulfur concentrations in the precursor, and how these variations change the surface morphology, crystal structure, electrical and optical properties, and solar cell performance of the thin film.
Sulfur vacancies, among other defects, may be another problem in sulfide-based thin film solar cells. A few papers have reported on ways to overcome the deficiency of sulfur in the fabrication of these materials, such as post-sulfurization, doping of thiourea, surface passivation, and maintaining a sulfur environment.19,20,21,22 Along these lines, we have increased the concentration of sulfur in the precursor to address the possible sulfur deficiency in the fabrication of Bi2S3 thin film.
Experimental
Chemicals and Materials
Chemicals and materials were obtained as follows: bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O) (purity ≥ 99.5%), thiourea (purity ≥ 98.0%), hydrochloric acid (HCL), 2-propanol, and toluene (99.5%, C6H5CH3 = 9.14) from Nacalai Tesque Inc.; 2-Me (2-methoxyethanol, purity ≥ 99.0%), titanium diisopropoxide bis(acetylacetonate), and poly(3-hexylthiophene-2,5-diyl) (P3HT, 99.995%) from Sigma-Aldrich; o-dichlorobenzene (99%), ethanol (EtOH 99.5%), and acetone (99.8%) from Kanto Chemical Co., Inc.; Triton X-100 from Sigma-Aldrich; FTO and 1-butanol from Kanto Chemical Co. Inc.; PELCO colloidal graphite from Ted Pella, Inc.; silver conductive paste from Fujiura Kasai Co.; Ltd. (Shanghai, China); and TiO2(P25) from Nippon Aerosil Co., Ltd.
Preparation of Electron Transport Layer (ETL)
For the preparation of the ETL, 2 cm \(\times \) 2 cm FTO substrates with a sheet resistance of 10 Ω/sq were ultrasonically cleaned in acetone and then in ethanol for 10 min. Then the cleaned FTO substrate was dried using a nitrogen gun. The dried substrate was treated with an ozone cleaner (Filgen Ozone Cleaner UV253-OZ) for 15 min. For the compact TiO2, 60 µl of titanium diisopropoxide bis(acetylacetonate) was diluted with 1 ml of 1-butanol, and the solution was maintained on a magnetic stirrer for 6 h at 600 rpm. The solution (80 µl) was dropped onto the FTO substrate and allowed to spin at 1500 rpm for 30 s using the spin coater device (Mikasa Opticoat Spin Coater MS-A100). Then, the sample was pre-annealed at 100°C for 10 min and post-annealed in a heating furnace at 400°C for 1 h in an air atmosphere. Similarly, for the mesoporous TiO2, P-25 powder (0.1 g), HCl (1 drop ≈ 40 µl), and Triton X-100 (1 drop ≈ 40 µl) were mixed in 2-propanol (2.5 ml) and maintained on a magnetic stirrer for 12 h. This solution was dropped onto a C-TiO2/FTO sample and spun at 2500 rpm for 30 s in air, after which it was annealed in a heating furnace at 450°C for 90 min.23
Preparation of Bi2S3 Thin Film
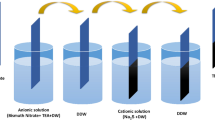
For the preparation of the Bi2S3 thin film, 303.168 mg Bi (NO3)3·5H2O, 1 ml of 2-Me (2-methoxyethanol), and thiourea were mixed.24 The mass of thiourea was adjusted such that the molar ratios of Bi to S in the solution were 1:1.0 (S1), 1:1.5 (S2), 1:2.0 (S3), 1:2.5 (S4), 1:3.0 (S5), and 1:3.5 (S6), respectively. Then the solution was shaken by a hand for 5 min, which revealed a color change in the solution from yellow to orange with the increased sulfur content, as shown in Fig. 1. Two drops (80 \(\mu {\text{l}})\) of the mixed solution were added to the m-TiO2/c-TiO2/FTO sample and spun at 2500 rpm for 30 s followed by low-temperature pre-annealing on a hotplate (AS ONE 1-6032-02 MMF-2) at 100°C for 5 min and high-temperature post-annealing on the same hotplate at 300°C for 15 min in a glovebox with a nitrogen atmosphere. The same process was carried out for the preparation of other samples.
Preparation of Hole Transport Layer (HTL)
For the preparation of the HTL, 15 mg of P3HT was dissolved in 1 ml of dichlorobenzene and maintained on a magnetic stirrer for 24 h at 60°C. The mixture was then deposited on Bi2S3 thin film at 3000 rpm for 20 s by spin coating followed by annealing on a hotplate at 120°C for 15 min in a glovebox with nitrogen gas.25
Preparation of Carbon Paste and Silver Electrode
Carbon paste (0.1 g) was diluted in 1.5 ml of 2-propanol23 and the mixture was maintained on a magnetic stirrer at 600 rpm for 6 h at room temperature. Drops (80 µl) of carbon solution were added to the sample and spun at 3000 rpm for 30 s in air and annealed at 100°C for 15 min. A small drop of silver paste diluted in toluene was placed on the carbon thin film, which was then dried at 80°C for 10 min. After all the work mentioned above, the sample was ready for I–V characterization. The schematic of the sample structure is shown in Fig. 2, and the active area is 0.07 cm2.
Characterization
The crystallinity and structure of Bi2S3 were characterized by x-ray diffraction (XRD, Rigaku RINT-2100) with a diffraction 2θ angle range from 10° to 80°. The surface morphology and EDS data were examined by field emission scanning electron microscopy (FE-SEM, JEOL JSM-7001F). The optical properties of the material in the UV–visible range were measured by UV-Vis spectrometry (Jasco V-570). The solar cell performance with different bismuth-to-sulfur ratios was measured under simulated solar light illumination (AM1.5, 100 mW cm−2) and a Keithley 238 light current source measure unit with a xenon lamp (Jasco, XCS-150) power supply sun simulator (Jasco, PS-X500).
Results and Discussion
XRD Analysis
Figure 3 shows the XRD pattern of Bi2S3 films for different Bi-to-S ratios. Bi2S3 has an orthorhombic crystal structure (a = 11.305 Ȧ, b = 3.981 Ȧ, c = 11.147 Ȧ) with space group pbnm.8,26 It has four molecules per unit cell, and each molecule contains two bismuth atoms and three sulfide atoms, which add up to 20 atoms per unit cell.26 The diffraction peaks at 2θ = 15.86°, 17.72°, 22.54°, 25.24°, 28.64°, 31.98°, 35.82°, 46.78°, and 52.7° are related to lattice planes (020), (120), (220), (130), (211), (221), (240), (002), and (351), confirming the deposition of Bi2S3 thin film in samples.24,27 The diffraction peaks at 2θ = 33.88°, 38.1°, 51.8°, and 61.92° corresponding to (101), (200), (211), and (310) lattice planes show the peaks of FTO.28 Similarly, two main intensity peaks of TiO2 are seen at 2θ = 25.28° and 48.02°.29 The XRD intensity peaks related to Bi2S3 are not seen in the S1 sample (Bi:S = 1:1), which is consistent with the amorphous form of the Bi2S3 thin film. However, a plane emerging at a broad peak (221) indicates the development of poor crystallization in sample S2, i.e., Bi2S3 material is not crystalized properly in samples of low sulfur concentration in the precursor. Furthermore, in sample S1, the supply stoichiometric ratio of S:Bi is less than the chemical stoichiometric ratio of Bi2S3 (1.5); hence, the sulfur deficiency might be explicitly reflected in crystal formation. But in samples of higher sulfur concentration in the precursor, the strong XRD peaks at 2θ = 25.24° and 28.64° reveal the crystalline structure of the material, and these peaks also matched with the powder diffraction standard of JSCPD 00-017-0320.3,4,30 This proves that the Bi2S3 has been successfully deposited on the surface of the m-TiO2 in these samples. The intensities of XRD peaks increase from samples S1 to S3 and remain nearly the same in sample solutions with higher concentrations of sulfur. But in sample S6 (S:Bi = 3.5), the XRD peak (211) corresponding to 2θ = 28.64° is relatively increased compared to the main peak at (130), signifying the starting of a phase change or crystal dislocation due to the excess sulfur, i.e., excess sulfur is detrimental to the lattice formation and crystal growth.20,21,22 The XRD main peak intensity of samples S1 and S2 lies towards the main peaks of the TiO2 and decreases in higher concentrations, which is shown in the enlarged inset in Fig. 3. This confirms that the crystallinity of the film is improved with increased sulfur content. Indeed, the strong and sharp XRD peaks at 2θ = 22.54°, 25.24°, and 28.64° reveal the high crystallinity of the Bi2S3 material in samples S3, S4, S5, and S6. Impurity peaks such as bismuth oxide and bismuth metals are not seen clearly in all samples, so these samples may not contain impurities. In the S1 sample, there is insufficient sulfur to form the crystal of Bi2S3 material, and a sulfur vacancy is formed due to high annealing temperature, resulting in increased sulfur deficiency for the crystallization.9,20 Table I shows the crystallite size of Bi2S3 calculated by using the Debye–Scherrer equation.
where \(\beta \) is the full width at half-maximum (FWHM) of the observed diffraction line, k is the shape factor (equal to 0.98), λ is the wavelength of the x-ray source of XRD, and θ is the diffraction angle for the maximum intensity. The crystallite size decreases with increasing Bi-to-S ratio, which will be discussed in the next section with SEM results.
Morphological Analysis
Figure 4 shows the FE-SEM surface view of Bi2S3 thin films for different Bi-to-S ratios. From FE-SEM data, for the film with a low concentration of sulfur, i.e., sample S1, nano-spherical particles with average diameter of 21 nm were found,4,11 and similar spherical particles with a diameter of 100 nm have been reported.31 In samples S2 and S3, a mixed surface morphology of nano-spherical and nanorod particles is found. This mixed morphology reveals that nano-spherical particles are aggregated to form nanorods with sharp edges that are randomly oriented on the surface of TiO2.32,33 The spherical particle with an average diameter of 25 nm and nanorod with length of 70 nm are seen in sample S2, which was measured using the Image J software program. Similarly, nanorods of longer length and smaller diameter are found in sample S4, and those nanorods cover the whole surface of m-TiO2. This shows the complete aggregation of nano-spherical particles to nanorods in sample S4. The density of nanorods increases continuously, forming a nanowire network. Almost the entire surface of the m-TiO2 is covered by nanowires of Bi2S3 in samples with a high concentration of sulfur. As the sulfur concentration increases, the length and diameter of these nanowires also increase, as shown for samples S5 and S6 in Fig. 4. The main peak of TiO2 at 2θ = 25.28° has almost vanished in samples with a high concentration of sulfur, which helps to confirm full coverage of TiO2 shown in Fig. 4. In sample S6, however, the nanowire density decreases, and the m-TiO2 layer does not appear to be entirely covered by nanowires. On the other hand, the crystallite size was slightly decreased initially and remained nearly the same in the samples with high concentrations of sulfur in the solution. The crystallite size was calculated as 12–17.25 nm, shown in Table I, and similar crystallite sizes are found in other published reports.24,34 The crystallite size of Bi2S3 depends on the types of synthesis and precursors.4,24,33
Table II and supplementary Fig. S1 show the surface EDS mapping and spectrum results for Bi2S3 films for different Bi-to-S ratios, indicating that the distribution of S, Bi, and Ti is almost uniform, but some pinholes in mesoporous TiO2 are seen especially in samples S2 and S3 due to the nonuniform size of grains in the mesoporous TiO2 solution, which also hinders solar cell efficiency. Some pinholes are covered by Bi2S3 nanowires in samples S5 and S6 because of the large size of the nanowires of Bi2S3, which is a more favorable condition for PCE. The S/Bi atomic ratios of Bi2S3 are shown in Table II. The S/Bi ratio increases from S1 to S3, and saturates to around 0.90–1.11. There is little difference between S3 and S6. From these data, we can say that excess sulfur changes the nanorods into nanowires in the thin film. Similar kinds of changes in morphology are reported in different papers.20,33 The shape and size of the nanoparticles evolve from nano-spherical to nanorod and then nanowires with increasing sulfur concentration, which is almost a new result in the spin coating method. The concentration of sulfur ions is low in sample S1, leading to the low reaction rate of the nucleation of Bi2S3. This low reaction rate facilitates the isotopic growth of Bi2S3 crystal, resulting in the formation of spherical nanoparticles. In sample S2, the availability of the sulfur ions increases and the reaction rate also increases, which facilitates the anisotropic growth of the crystal, leading to the nanorod structure. Similarly, a further increase in the concentration of sulfur ions produces more anisotropic growth, resulting in nanowire formation in samples S3 and S4 and larger nanowires in S5 and S6.20,34 Different shapes and sizes of Bi2S3 particles are reported such as nanostars, nanoballs, nanoflakes, nanorods, nanoflowers, nanobelts, nanotubes, and nanowires depending on the synthesis method and nature of precursors.9 Interestingly, our research shows that the shape and size of the particles of Bi2S3 material also depend on the richness of sulfur content in the solution. Furthermore, the size of the grain increases with the further increase in the concentration of sulfur in the solution of the sample, but the crystallite size remains nearly the same in samples with high sulfur concentrations, namely samples S4, S5, and S6. The crystallite size and grain size are independent of each other, i.e., the same crystallite size does not necessarily mean that the grain size is the same.24 This result reveals that the morphology of Bi2S3 material not only depends on annealing temperature, method of synthesis, nature of precursors, and atmosphere during the synthesis, but also depends on the richness of the sulfur content in the sample solutions, which is a unique result of our study. The surface of Bi2S3 film is not uniform, so some pin holes are seen in solution samples with low sulfur concentration. However, in high-concentration sulfur samples, fewer pin holes are seen due to the presence of larger gain size and might also increase the thickness of Bi2S3.35 Sulfur deficiency is seen in the S1 sample, so the number of Bi molecules dominates in thin film formation and becomes almost saturated in high-concentration sulfur samples.
Figure 5 shows the typical cross-sectional view for Bi2S3 at a Bi-to-S ratio of 1:3.0 (S5). A separate Bi2S3 film (around 150 nm) and a Bi2S3/TiO2 composite film (around 400 nm) are observed. Bi2S3 covers the surface of the TiO2, which is seen in the cross-sectional and SEM images. It is also clear from the EDS results that Bi2S3 material is present only on the substrate; however, Bi is more abundant than S in all samples, which is confirmed by supplementary Fig. S1 and Table II. The dominant role of Bi in thin film formation generates strong sulfur deficiency defects and the structural defect phenomenon in samples.20,22
Optical Analysis
The absorption spectra of the samples for different Bi-to-S ratios are shown in Fig. 6. The absorption edge is shifted to the longer wavelength with the increasing concentration of sulfur in the sample solution. Consequently, the band gap is shifted to a lower value, which is a favorable condition for the photoelectric effect. The optical band gap is calculated using the Tauc plot formula of (αhν)2 versus hν, where α is the absorption coefficient, h is the Planck constant, and ν is the frequency of incident light as shown in Fig. 7. The band gap varies with the sulfur concentration in solution from 1.86 eV to 1.48 eV, as shown in Table III, and similar types of variation have been reported in other papers.21,22 The variation in the band gap is dependent on the nature of precursors, synthesis method, annealing temperature, and surface morphology.11,36,37 On average, the band gap is decreased in thin film with high concentrations of sulfur. In sulfide-based compounds, the concentration of sulfur content plays a major role in energy band structure formation by shifting of the valence band edge as a result of the change in band gap value. The stoichiometry ratio of S:Bi is almost constant in samples with high concentrations of sulfur; however, the excess sulfur facilitates the change in the grain boundaries, which is also seen in SEM data. That change in gain boundary and mid-gap defect states due to sulfur deficiency are responsible for the band gap change. Hence, the excess sulfur tends to decrease the band gap by reducing possible defects.20,21,22
As mentioned in the XRD analysis and morphology analysis sections, Bi2S3 was transformed from an amorphous-like spherical nanoparticle (S1 and S2) to a crystalline nanorod (S3 and S4), and finally to a crystalline nanowire (S5 and S6). These features correlate with the value of the band gap, i.e., ~ 1.8 eV for S1 and S2, ~ 1.6 eV for S3 and S4, and ~ 1.5 eV for S5 and S6. Some possible point defects are reduced due to the increased concentration of sulfur in the solution; consequently, particle size is increased and a more uniform film is formed. The thickness of the Bi2S3 thin film may also be increased. The defect density reduction is related to the improvement of solar cell performance, which will be described in the next section. Similarly, when the concentration of sulfur is increased, the crystallize size is decreased, resulting in a decrease in the band gap.20
Photovoltaic Analysis
In our work, the FTO/c-TiO2/m-TiO2/Bi2S3/P3HT/carbon/Ag structure as shown in Fig. 2 was studied for solar cell analysis. The current–voltage curves of different molar ratios of Bi and S are shown in Fig. 8. The short-circuit current (Jsc), open-circuit voltage (Voc), fill factor, and PCE are summarized in Table III. The short-circuit current (Jsc) and open-circuit voltage (Voc) are increased with the increasing concentration of sulfur in the solution up to S5 (Bi:S = 1:3.0), resulting in maximum PCE. However, in sample S6 (Bi:S = 1:3.5), Voc is decreased significantly, which indicates that excess sulfur is detrimental to the thin film solar cell performance.20,28,38 As previously mentioned, when Bi2S3 material is spread onto m-TiO2, Bi2S3 layers and TiO2/Bi2S3 composite layers are formed as shown in Fig. 5. The improvement in solar cell performance from S1 to S5 is due to the morphological change from spherical nanoparticle to nanowire, as shown in Fig. 4, and the structural change from amorphous to crystalline, as shown in Fig. 3. On the other hand, the solar cell performance is greatly improved with the nanowire structure as explained below. The photogenerated carrier moves much more easily in crystalline nanowires than in nanoparticles. High photoconductivity is reported in Bi2S3 nanowires.17 Therefore, a reduction in series resistance is expected with nanowires. Another reason for the improvement is the formation of the TiO2/Bi2S3 nanowire composite as shown in Fig. 5. When photons are absorbed in Bi2S3 nanowires, forming electron–hole pairs, the hole can easily move towards the HTL through the nanowire network. In contrast, the electron can move towards the ETL through the porous TiO2 network. The drop in solar cell performance in sample S6 with an excessively high sulfur concentration in the solution is due to the decrease in nanowire density, resulting in the large gap between nanowires, as shown in Fig.4 (S6). Carbon and silver electrodes can easily penetrate the TiO2 layer through the gap, resulting in the reduction in parallel resistance.
Discussion
In this paper, it was shown that the spin-coated Bi2S3 transforms from nanoparticle films to nanowire films with the increase in the sulfur-to-bismuth ratio of the solution. First, this change is briefly described. The formation of Bi2S3 from Bi(NO3)3 and thiourea (TU) in the solvent is expressed as follows.39
It is expected that n (number of molecules) increases with the increasing sulfur-to-bismuth ratio, as confirmed by the change in the color of the solution, shown in Fig. 1. A vapor-liquid-solid (VLS) model is used to explain the growth of Bi2S3 nanowires as a synthesis of other oxide nanowires.40 In the case of a low sulfur-to-bismuth ratio, nanoparticle films are formed. However, with an increasing ratio, the interactions of Bi (TU)n3+ molecules are enhanced; therefore, once small Bi2S3 nuclei are formed, the growth of long nanowires takes place via the VLS model.
We studied the structural, morphological, optical, and electrical properties of the thin film due to the variation in sulfur ion concentration in the precursor solution. The sulfur-rich condition facilitates the environment for crystal formation as well as the growth of the nanoparticles from spherical to nanorods and then finally to nanowires, as shown in Fig. 4. The continuous increase in the second main intensity XRD peak at the (211) plane (2θ = 28.64°) indicates the development of a preferable environment for the crystal formation of Bi2S3 material. Similarly, the generation of new peaks at (020), (120), (220), and (221) confirms the continuous improvement in crystal formation in samples with increased concentrations of sulfur in the precursor solution, as shown in Fig. 3. Furthermore, the length and diameter of the nanowire increase with increasing sulfur content in the solution of samples S3–S6, as shown in Fig. 4, but in sample S2, nanorods are found. A similar morphological change from nanorods to nanowires with the increasing S-to-Bi ratio has been reported.24 The continuous increase in the shape and size of nanowires and decrease in mesoporous TiO2 pinholes collectively enhance the increasing carrier concentration in samples S3, S4, and S5, which also corresponds to the increased intensity peaks in the XRD data at 2θ = 25.24° and 28.64°. The decrease in the nanowire density and the intensity of the XRD peaks at 2θ = 15.86° and 17.72° in sample S6 explicitly shows that excess sulfur influences the density of the nanowires as well as the crystal orientation. The increased nanowire size, especially in sample S6, increases surface roughness, which increases the effective interfacial area between the Bi2S3 layer and both the ETL and HTL, resulting in an increase in the concentration of traps and consequently damping the photocurrent.38,41 The surface of the TiO2 is not fully covered by the Bi2S3 particles in samples S1, S2, and S3; therefore, some incident photons fall directly on TiO2 nanoparticles, and TiO2 nanoparticles exhibit a relatively low photoresponse to incident light due to its high band gap, resulting in a decrease in PCE even though photon absorption remains nearly the same.42
Various defects including sulfur vacancy, surface, bulk, and interfacial defects also affect the solar cell performance overall.8,35 Among them, sulfur vacancy defects might be the main cause of low photocurrent generation in all samples due to thermal decomposition with the high annealing temperatures and the more volatile nature of sulfur, which induces deep traps for the recombination of charge carriers.19,20 In particular, in sample S1, both sulfur vacancies and low availability of sulfur lower the photocurrent generation. Similarly, excess sulfur conditions may produce by-products such as H2S NH3 and some impurities as well as an increase in occupancy of Bi at S interstitial sites.20,21,22 As a result, both voltage and current may decrease suddenly in sample S6. Furthermore, sulfur is a polyatomic nonmetal and it can act as a barrier to charge flow in photoconduction in film with high concentrations of sulfur. Similarly, sulfur vacancies facilitate deep traps or other point defects, which is an n-type defect located at mid-gap. These are some major defects resulting in the poor solar cell performance of the Bi2S3 thin film.43 The high annealing temperature (300°C) could be another important reason for the low sulfur content in the samples,19,20,21 and the effect of oxygen atoms may increase to produce an oxide family of bismuth atoms.
The continuous increase in sulfur in the solution may increase the conductivity and the absorption range of photo-irradiation in the corresponding thin films, resulting in greater photogeneration in the thin film, as shown in the I–V graph in sample S5. Overall, high sulfur concentration in the precursor solution shows an improvement in crystallization and surface morphology, and decreased band gap value, which correlate with the improvement in I–V results; hence, sample S5 (Bi:S = 1:3) shows higher open-circuit voltage and short-circuit current, resulting in maximum efficiency. The corresponding ratio is the optimized ratio of Bi to S in the precursor solution for Bi2S3 thin film. A similar kind of optimized ratio of Bi to S is reported in SbSI thin film.44 The stoichiometric ratio of S to Bi in the samples is almost 0.9, which is much less than the ideal value of 1.5, and this may be another major reason for the low efficiency in our work, as the study of close stoichiometric ratio data has shown greater efficiency.3,4 This low stoichiometric ratio of S:Bi might be due to the nature of the precursors or the synthesis method, which arose as the main problem in our work. However, increasing the concentration of sulfur in the solution overcomes this issue to some extent. The best efficiency of the solar cell is obtained by the integration of factors like the thickness of the film, carrier lifetime, absorption coefficient, and conductivity. However, the supply of excess sulfur in the precursor solution might not completely address the possible sulfur deficiency in the synthesis process alone. Hence, low-temperature synthesis, noble synthesis, and the optimization of all individual factors are necessary for further improvement.
Conclusion
Bi2S3 nanowire thin film solar cells were fabricated on m-TiO2/c-TiO2/FTO glass substrate at a relatively high sulfur-to-bismuth ratio in the precursor by spin coating. We investigated the influence of the sulfur concentration in the solution on the structural, morphological, electrical, and optical properties of the Bi2S3 thin film. The XRD analysis and EDS data for the thin films confirm the formation of a nano-orthorhombic crystalline structure of Bi2S3 on the surface of TiO2. The morphology of the thin film continuously evolves with the concentration of sulfur in the solution, with spherical nanoparticles in low concentrations gradually becoming nanorods and then nanowires in high concentrations. The length of the nanorod is also increased with the increasing concentration of sulfur. The atomic ratio of S and Bi in the thin film becomes almost constant at 0.9 except for the sample with the low sulfur concentration in the solution. In high concentrations of sulfur, Bi2S3 nanowires cover the TiO2 surface. The band gap of the samples decreases from ~ 1.9 to ~ 1.5 eV. Both Jsc and Voc and solar cell efficiency are greatest in the Bi:S = 1:3 sample; thus the 1:3 ratio represented the optimized stoichiometric ratio of Bi to S in Bi2S3 thin film for solar cell performance. This shows that the Bi2S3 nanowire is a promising material for future solar cell fabrication.
References
J.Y. Kim, J.W. Lee, H.S. Jung, H. Shin, and N.G. Park, High-efficiency perovskite solar cells. Chem. Rev. 120, 7867 (2020).
S. Wang, Y. Zhao, B. Che, C. Li, X. Chen, R. Tang, J. Gong, X. Wang, G. Chen, T. Chen, J. Li, and X. Xiao, A novel multi-sulfur source collaborative chemical bath deposition technology enables 8%-efficiency Sb2S3 planar solar cells. Adv. Mater. 34, 2206242 (2022).
M. Koltsov, S.V. Gopi, T. Raadik, J. Krustok, R. Josepson, R. Gržibovskis, A. Vembris, and N. Spalatu, Development of Bi2S3 thin film solar cells by close-spaced sublimation and analysis of absorber bulk defects via in-depth photoluminescence analysis. Sol. Energy Mater. Sol. Cells 254, 112292 (2023).
P.M. Mathato, D.C. Onwudiwe, and L. Wei, Microwave-assisted synthesis of Bi2S3 and Sb2S3 nanoparticles and their photoelectrochemical properties. ACS Omega 6, 18975 (2021).
C. Nichole and B. María, Research update: bismuth based materials for photovoltaics. APL Mater. 6, 084503 (2018).
J. Arumugam, A.D. Raj, A.A. Irudayaraj, and M. Thambidurai, Solvothermal synthesis of Bi2S3 nanoparticles and nanorods towards solar cell application. Mater. Lett. 220, 28 (2018).
J. Arumugam, A.D. Raj, and A.A. Irudayaraj, Reaction time dependent investigation on the properties of the Bi2S3 nanoparticles: photocatalytic application. Mater. Today Proc. 5, 16094 (2018).
Z. Li and Y. Tian, Nano-bismuth-sulfide for advanced optoelectronics. Photonics. 9, 790 (2022).
T.O. Ajiboye and D.C. Onwudiwe, Bismuth sulfide based compounds, properties, synthesis and applications. Results Chem. 3, 100151 (2021).
N. Mahuli, D. Saha, and S.K. Sarkar, Atomic layer deposition of p-type Bi2S3. J. Phys. Chem. C 121, 8136 (2017).
D.C. Onwudiwe and V.M. Nkwe, Morphological variations in Bi2S3 nanoparticles synthesized by using a single source precursor. Heliyon. 6, 1 (2020).
H.M. García, M.T.S. Nair, and P.K. Nair, Chemically deposited lead sulfide and bismuth sulfide thin films and Bi2S3/PbS solar cells. Thin Solid Films 519, 2287 (2011).
H. Song, X. Zhan, D. Li, Y. Zhou, B. Yang, K. Zeng, J. Zhong, X. Miao, and J. Tang, Rapid thermal evaporation of Bi3S3 layer for thin film photovoltaics. Sol. Energy Mater. Sol. Cells 146, 1 (2016).
A.S. Rasal, K. Dehvari, G. Getachew, C. Korupalli, A.V. Ghule, and J.Y. Chang, Efficient quantum dot-sensitized solar cells through sulfur-rich carbon nitride modified electrolytes. Nanoscale 13, 5730 (2021).
M. Han and J. Jia, 3D Bi2S3/TiO2 cross-linked heterostructure: an efficient strategy to improve charge transport and separation for high photoelectrochemical performance. J. Power. Sources 329, 23 (2016).
R. Nishikubo and A. Saeki, Solution-processed Bi2S3 photoresistor film to mitigate a trade-off between morphology and electronic properties. J. Phys. Chem. Lett. 9, 5392 (2018).
J. Andzane, G. Kunakova, J. Varghese, J.D. Holmes, and D. Erts, Photoconductive properties of Bi2S3 nanowires. J. Appl. Phys. 117, 064305 (2015).
J. Arumugam, A. George, A.D. Raj, A.A. Irudayaraj, R.L. Josephine, S.J. Sundaram, A.M. Al-Mohaimeed, W.A. Al-onazi, M.S. Elshikh, and K. Kaviyarasu, Construction and characterization of photodiodes prepared with Bi2S3 nanowires. J. Alloys Compd. 863, 158681 (2021).
A. Kamble, B. Sinha, G. Agawane, S. Vanalakar, I.Y. Kim, J.Y. Kim, S.S. Kale, P. Patil, and J.H. Kim, Sulfur ion concentration dependent morphological evolution of CdS thin films and its subsequent effect on photo-electrochemical performance. Phys. Chem. Chem. Phys. 18, 28024 (2016).
J. Li, X. Liu, and J. Yao, The enhanced photovoltaic performance of Sb2S3 solar cells by thermal decomposition of antimony ethyl xanthate with thiourea doping. Energy Technol. 8, 1900841 (2020).
H. Sun, Y. Xue, L. Wang, Q. Guo, and P. Li, Research on the properties of ZnO1-xSx thin films modified by sulfur doping for CIGS solar cells. Optoelectron. Lett. 18, 678 (2022).
L. Beddek, M. Messaoudi, N. Attaf, M.S. Aida, and J. Bougdira, Sulfide precursor concentration and lead source effect on PbS thin films properties. J. Alloys Compd. 666, 327 (2016).
M.M. Rahaman, M.A.A. Noman, S. Kato, and T. Soga, A novel modest synthesis of device applicable flakes based stable BiOI film by the oxidation of BiI3 film. J. Alloys Compd. 873, 159715 (2021).
Z. Jia, M. Righetto, Y. Yang, C. Chelsea, Y. Li, R. Li, Y. Li, B. Yu, Y. Liu, H. Yong, H. Huang, M. Johnston, L. Herz, and Q. Lin, Charge-carrier dynamics of solution-processed antimony- and bismuth-based chalcogenide thin films. ACS Energy Lett. 8, 1485 (2023).
M.F. Achoi, M.A.A. Noman, S. Kato, N. Kishi, and T. Soga, Pinhole-free methylammonium bismuth iodide perovskite solar cells via all-solution-processed multi-step spin coating. J. Electron. Mater. 51, 577 (2022).
H. Bao, X. Cui, C.M. Li, Y. Gan, J. Zhang, and J. Guo, Photo switchable semiconductor bismuth sulfide (Bi2S3) nanowires and their self-supported nanowire arrays. J. Phys. Chem. C 111, 12279 (2007).
F. Ding, Q. Wang, S. Zhou, G. Zhao, Y. Ye, and R. Ghomashchi, Synthesis of Bi2S3 thin films based on pulse-plating bismuth nanocrystallines and its photoelectrochemical properties. R. Soc. Open Sci. 7, 200479 (2020).
H.Z. Asl and S.M. Rozati, High-quality spray-deposited fluorine-doped tin oxide: effect of film thickness on structural, morphological, electrical, and optical properties. Appl. Phys. A 125, 689 (2019).
M. Shaban, J. Poostforooshan, and A. Weber, Surface-initiated polymerization on unmodified inorganic semiconductor nanoparticles via surfactant-free aerosol–based synthesis toward core-shell nanohybrids with tunable shell thickness. J. Mater. Chem. A 5, 18651 (2017).
M. Khadraoui, B. Noureddine, R. Miloua, C. Mathieu, A. Bouzidi, and K. Sahraoui, Optical properties of sprayed Bi2S3 nanocrystalline thin film. Optoelectron. Adv. Mat. 9, 1167 (2015).
S.K. Ambre, S.S. Patil, T.S. Bhat, and P.S. Patil, Bi2S3-sensitized TiO2 nanorods by bottom-up approach for photoelectrochemical solar cell. Solid State Electrochem. 27, 337 (2023).
S. Wang, J. Li, Y. Fu, Z. Zhuang, and Z. Liu, Indium-doped mesoporous Bi2S3 -based electrochemical interface for highly sensitive detection of Pb (II). Microchem. J. 166, 106251 (2021).
C. Sambathkumar, V. Manirathinam, A. Manikandan, M. Krishna Kumar, S. Sadhahar, and P. Devendran, Solvothermal synthesis of Bi2S3 nanoparticles for active photocatalytic and energy storage device applications. J. Mater. Sci. Mater. Electron. 32, 1 (2021).
A. Begum, A. Hussain, and A. Rahman, Preparation and characterization of bismuth sulphide nanocrystalline thin films by chemical bath deposition method in acidic aqueous media. Chalcogenide Lett. 8, 283 (2011).
M.A. Moghri Moazzen, S.M. Borghei, and F. Taleshi, Change in the morphology of ZnO nanoparticles upon changing the reactant concentration. Appl. Nanosci. 3, 295 (2013).
D. Zhu, X. Li, Y. Chen, Y. Deng, D. Chen, Y. Wang, and F. Ren, Effect of Bi2S3 reaction concentration on the photoelectric properties of TiO2/Bi2S3 composite film. Mater. Res. Express. 6, 095026 (2019).
L. Ma, Q. Zhao, Q. Zhang, D. Meng, J. Huang, X. Liu, Y. Liu, X. Wu, and X. Xu, Controlled assembly of Bi2S3 architectures as Schottky diode, supercapacitor electrodes and highly efficient photocatalysts. RSC Adv. 4, 41636 (2014).
A. Maiti, S. Chatterjee, and A.J. Pal, Sulfur-vacancy passivation in solution-processed Sb2S3 thin-films: influence on photovoltaic-interfaces. ACS Appl. Energy Mater. 3, 810 (2020).
S.B. Erenburg, S.V. Trubina, and N.N. Golovnev, Structure of bismuth (III) complexes with thiourea according to EXAFS spectroscopy Data. Russ. J. Phys. Chem. 87, 461 (2013).
A. Mirzaei, M.H. Lee, K.K. Pawar, S.P. Bharath, T.-U. Kim, J.-Y. Kim, S.S. Kim, and H.W. Kim, Metal oxide nanowires grown by a vapor–liquid–solid growth mechanism for resistive gas-sensing applications: an overview. Mater. 16, 6233 (2023).
Z. Ma, Z. Tang, E. Wang, M.R. Andersson, O. Inganäs, and F. Zhang, Influences of surface roughness of ZnO electron transport layer on the photovoltaic performance of organic inverted solar cells. J. Phys. Chem. C 116, 24462 (2012).
F.G. Cai, F. Yang, Y.F. Jia, C. Ke, C.H. Cheng, and Y. Zhao, Bi2S3-modified TiO2 nanotube arrays: easy fabrication of heterostructure and effective enhancement of photoelectrochemical property. J. Mater. Sci. 48, 6001 (2013).
Y.C. Choi, E. Hwang, and D.H. Kim, Controlled growth of SbSI thin films from amorphous Sb2S3 for low-temperature solution processed chalcohalide solar cells. APL Mater. 6, 121108 (2018).
R. Kondrotas, C. Chen, and J. Tang, Sb2S3 solar cells. Joule. 2, 857 (2018).
Funding
Open Access funding provided by Nagoya Institute of Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khadka, D.B., Islam, M.A., Kato, S. et al. Fabrication and Properties of Bi2S3 Nanowire Thin Film Solar Cells by Spin Coating with Varying Sulfur Concentrations in the Precursor. J. Electron. Mater. 53, 4224–4235 (2024). https://doi.org/10.1007/s11664-024-11129-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-11129-2