Abstract
We present the results of the photoluminescence behavior reflecting Cr2+ → Fe2+ excitation transfer in co-doped ZnSe:Cr2+Fe2+. This transfer can be seen as a possible promising pump mechanism to create short pulse lasers for the 3- to 6-µm wavelengths that can be excited using inexpensive 2-µm pump light sources. In addition to the kinetics, emphasis was put on comparing the intensities of both emissions, those of Cr2+ and Fe2+. With resonant excitation of Cr2+, the kinetics of the Fe2+ emission shows a very clear picture of the transfer with 60-ns rise time and the peak delayed by 200 ns. However, the evaluation of the PL intensities brought a surprise. With efficiencies above 80%, the observed Cr2+ → Fe2+ transfer is much more efficient than theoretically expected, and even the overall efficiency including the losses at the Fe2+ ions of almost 4% is still an order of magnitude higher than the theoretical values given for the Förster transfer alone. This leads to the suspicion that either the dopant atoms might not be uniformly distributed in the samples or that the transfer mechanism might be more effective than previously thought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cr2+ and Fe2+ defect centers located at Zn-sites in the zincblende lattice of ZnSe exhibit broad emission bands at 2.4 µm and 4.5 µm, respectively.1,2 Therefore, such classical II–VI semiconductor crystals appear ideal candidates for optically pumped solid-state lasers in the mid-infrared range. In particular, the phonon-broadened character of the emission bands is appealing for building mid-infrared mode-locked lasers with few-cycle pulse duration (see Fig. 1). Therefore, ZnSe:Cr2+ has frequently been dubbed as the Ti:sapphire of the mid-infrared. While commercial high-power Tm-doped fiber lasers are conveniently available for pumping ZnSe:Cr2+ lasers, there are no lasers offering the elevated pump power requirements of ZnSe:Fe2+ at 3.2 µm. Given this constraint, direct excitation of Cr2+ ions at 2.4 µm with subsequent Cr2+ → Fe2+ excitation transfer offers a promising alternative. This transfer has been the subject of several previous studies.3,4,5,6,7 Consistently, both experimental and theoretical studies showed such unsatisfactory results for the overall transfer efficiency that a practical use for this mechanism seemed quite unlikely. In a previous study,8 however, we observed a six-fold acceleration of the Cr2+ photoluminescence (PL) in co-doped ZnSe:Cr2+Fe2+ compared to a singly-doped reference sample. While this finding is highly suggestive of an efficient transfer process, the overall efficiency was again measured as a meager 3.6%. The aim of current work is the clarification of this apparent contradiction. Covering a wider range of doping concentrations, we augmented our time-resolved PL studies on singly- and co-doped crystals. Analysis of these experimental results confirms our earlier suspicion of a high Cr2+ → Fe2+ transfer efficiency, pinpointing non-radiative losses of excited Fe2+ ions as the source of the relatively poor overall efficiency. Mitigation strategies for practical laser applications involve cooling of the ZnSe:Cr2+Fe2+ laser crystals.
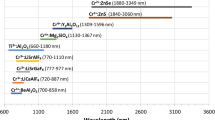
Normalized emission cross-sections of Cr2+ and Fe2+ ions in ZnSe3 , and the spectral windows used for the investigation (SP short pass, LP long pass); the brown line represents the transmission of 32 cm of air, the path the PL signal has from the sample to the detector (Color figure online)
Experimental
Our study comprises singly-doped ZnSe:Cr2+ and ZnSe:Fe2+a as well as co-doped ZnSe:Cr2+Fe2+ crystals with ion concentrations of 3 × 1016 cm−3…5.5 × 1018 cm−3, 4 × 1018 cm−3, and 3 × 1016 cm−3…7.5 × 1018 cm−3. These crystals were purchased from several commercial sources: Egorov Scientific US, 3photon Lithuania, and IPG Photonics US. The sample from the last source are grown by chemical vapor deposition,9 while the others are Bridgeman-grown singly crystals. We characterized the samples by standard transmission measurements at ambient temperature. Concentrations (N) of optically active Cr2+ and Fe2+ions were determined using the commonly accepted absorption cross-sections of 1.1 × 10–18 cm2 and 0.97 × 10–18 cm2 for Cr2+ and Fe2+ ions, respectively.3,10,11
The transient PL was excited with pulsed lasers, described in detail by Fürtjes et al.12 Excitation wavelengths were λex = 2.05 µm (pulse duration 3 ps) and λex = 3.24 µm (pulse duration 1 ps), using a repetition rate of 1 kHz. PL was detected with a LN2-cooled InSb-photodiode (Infrared Acc. ID413), enabling 7-ns temporal resolution. Using a Femto DHPCA-100 amplifier with 1-kV/A transimpedance, this temporal resolution remains almost preserved, i.e., we yielded sub-10-ns temporal resolution. Data recording was by a 4-GHz oscilloscope (DPO 70404C; Tektronix).
Emission intensities were extracted in spectral windows, named SP and LP, which are defined by sets of filters (Spectrogon; Edmund Optics). Figure 1 shows them together with the λex (arrows) and the normalized emission cross-sections of the Cr2+ and Fe2+ ions. Although absolute PL intensities cannot be measured, accurate consideration of the excitation, transfer, and detection conditions allows for relative comparison of the PL from the Cr2+ and Fe2+ ions in terms of photon numbers. In all the experiments, the time-averaged excitation power was set to ~ 35 mW, avoiding saturation excitation in both ion species.
Results and Discussion
Photoluminescence Kinetics
Figure 2 shows selected PL transients for resonant excitation. Under direct excitation, all the Fe2+-doped samples with ions in (b) exhibit a singly-exponential PL decay in the LP window, whereas the transients of the Cr2+ -containing samples exhibit different behaviors, measured in the corresponding SP window (Fig. 2a). Among the latter, all singly-doped samples show a strictly exponential decay, which is contrasted by the more or less pronounced non-exponential behavior of the co-doped samples. Such deviations from a singly exponential decay is a hallmark of excitation transfer processes.
PL transients for resonant excitation: (a) from ZnSe:Cr2+ and ZnSe:Cr2+Fe2+ co-doped samples excited λex = 2.05-µm and detected in the SP window, (b) from ZnSe:Fe2+ and ZnSe:Cr2+Fe2+ co-doped samples with 3.24-µm excitation and detected in the LP window; in (a) and (b), blue curves represent data from singly doped samples while data from co-doped samples are given in red (Color figure online)
Before deepening the discussion on Cr2+ → Fe2+ excitation transfer, let us briefly consider relative PL intensities. Under identical excitation conditions, the total PL emission yield of singly-doped Fe2+-doped samples is ~ 14 times lower than that of the Cr2+ PL. This finding already sets a constraint of ηFe ~ 7% for the maximum quantum efficiency of the Fe2+ PL. Further confirmation of this rather poor quantum efficiency can be found in the literature, specifically from temperature-dependent measurements of the PL decay time,13,14,15,16,17 indicating the practical absence of the temperature-quenching effects for the Cr2+ PL. While the PL decay time (τPL) of the Cr2+ ions remains nearly constant,13 the pronounced decrease of τPL of the Fe2+ ions from ~ 100 µs (at ~ 100 K) to ambient temperature by more than two orders of magnitude can be seen14,15,16,17 As temperature-quenching is always indicative of non-radiative decay processes, this gives rise to the observed degradation of the PL quantum efficiency of the Fe2+ ions.
Figure 3 shows τPL versus doping concentration, N, as obtained from the transients shown in Fig. 2. In the following discussion, we refer to the inter-ionic distance, R, which is shown as the top abscissa of Fig. 2 and is related to N via R = (1/N)1/3. As our sample set covers only a limited doping concentration range, we have additionally included literature data3,16,18,19 in Fig. 3. Both ion species exhibit concentration-quenching, i.e., excitation transfer between ions of identical species. However, the quenching effects only appear for N > 1019 cm−3, giving rise to a reduction of τPL. As our studies only comprised samples of lower doping, we can therefore safely rule out concentration-quenching. In agreement with previous reports, we also find that, in co-doped samples, the respective other dopant reduces the PL decay time of the studied species;3,4,18,20,21 compare positions of squares (singly-doped) and circles (co-doped) in both parts. The observed τPL reductions with N are empirically described by:
see, e.g.,16 where N0 is a fit parameter quantifying the concentration quenching threshold. The respective values N0 = 4 × 1019 cm−3 and 1.07 × 1020 cm−3 for Cr2+ and Fe2+ indicate that this process starts at lower doping levels for Cr2+. In addition, the general shape of this calculated curve agrees less well with the data points, in contrast to Fe2+, where the calculation and experiment are in better agreement. Overall, this analysis of the PL decay times provides remarkable agreement with data from the literature.
PL decay time versus ion concentrations for resonant excitation: (a) λex = 2.05 µm and detection in the SP window; blue symbols stem from fits to transients in the 10.0- to 12.5-µs temporal window (see Fig. 2a), black symbols are taken from figure 5 in Ref. 19 and see Refs. 13,27 (b) λex = 3.24 µm and detection in the LP window; red symbols are from fits to transients in the 0.25- to 1.0-µs temporal window (see Fig. 2(b); black, blue, and green symbols are data from Refs. 16, 4, and 3, respectively. In (a) and (b), squares and circles represent data from singly- and co-doped samples, respectively (Color figure online)
Figure 4 shows PL transients from heavily co-doped sample (NCr = 6.7 × 1018 cm−3 , NFe = 6.1 × 1018 cm−3) after λex = 2.05-µm excitation taken in the SP and LP windows. Extra measurements served to exclude a contribution of directly excited Fe2+ ions, making us conclude that the LP transient has its origin in the radiative recombination from Fe2+ ions that have been indirectly excited via Cr2+ ions. This assertion is confirmed by.
-
The rise time (~ 60 ns) of the transient, which substantially exceeds the time resolution (< 10 ns),
-
a delayed peak some 200 ns after the excitation laser peak, and
-
a significantly increased PL decay time of the Fe2+ PL of ~ 1 µs.
Normalized PL transients from 3 samples; blue transients show the PLs from a heavily co-doped sample (NCr = 6.7 × 1018 cm−3, NFe = 6.1 × 1018 cm−3) after λex = 2.05-µm excitation taken in the SP and LP windows; black and red curves serve for reference and have been obtained from singly-doped samples (Color figure online)
These experimental findings can be accurately explained by a rate equation model, which will be presented elsewhere.22 Qualitatively, the complex time evolution of the Fe2+ PL originates from a distribution of excitation transfer times, arising from a distribution of transfer distances in the ZnSe host crystal. At the shortest distances, the fastest processes result in the initial increase of the Fe2+ PL with a 60-ns rise time, while slower transfer processes cause Fe2+ ions to emit light much beyond their intrinsic PL decay time of 380 ns.
Photoluminescence Intensities
Apart from direct analysis of PL kinetics, it also appears interesting to compare the time-integrated PL intensities of the two ion species in co-doped samples for an estimation of the transfer efficiency. Figure 4 shows an example case, with an integrated intensity ratio of 1:0.03, i.e., the luminous efficiency of the Fe2+ ions is 33 times lower than that of the Cr2+ ions. Recalling that the radiative quantum efficiency is less than ηFe = 7%, one concludes that the Cr2+ → Fe2+ excitation transfer must be relatively efficient, with a value of ηTransfer = 83% for this particular sample. Figure 5 shows integrated intensity ratios of the two ion species versus Fe2+ concentration. For our most heavily Fe2+ -doped sample (NCr = 6.5 × 1018 cm−3, NFe = 7.5 × 1018 cm−3), we even conclude a transfer efficiency of ηTransfer = 87%.
(a) Cr2+ → Fe2+ excitation transfer efficiency calculated from γ according to Doroshenko et al.26; the right ordinate shows the integrated PL intensity ratios of Fe2+ to Cr2+ PLs for the same samples (see full red circles); the black line is a guide for the eye. (b) Förster transfer time (τF) according to Fedorov et al.3 (red line) and expected PL decay time taking into account τF and τCr = 5.5 µs (see Fig. 2a); see blue line versus interionic spacing; the full red circles are from table 1 in Fedorov et al.3 (c) Förster transfer efficiency ηF = τCr/(τCr + τF) versus Fe2+ concentration (Color figure online)
In the light of the previously discussed poor overall efficiency, nearly perfect transfer efficiencies appear highly surprising. In the consistent view of the literature on Förster transfer, dipole–dipole interaction appears as the key mechanism. The original publications by Förster23 and Dexter24 are summarized by Henderson and Imbusch,25 and were applied to the material system discussed here by Fedorov et al.3 Based on this theoretical approach, Doroshenko et al.26 estimated a similar high transfer efficiency of ηTransfer = 53–55% by analyzing the Cr2+ PL in their Zn1−xMnxSe:Cr2+Fe2+ samples. Specifically, the authors separated exponential and non-exponential contributions to their Cr2+ transients, and plotted the non-exponential part versus the square root of time. The slopes, γ, obtained from the linear fits of the data enabled an alternative method for estimating efficiencies. In order to verify our above conclusions, we also followed this approach, as shown in Fig. 5a with respect to the left ordinate.
Finally, we compared our PL decay times with the calculations by Fedorov et al.3 Figure 5b and c shows the time constants and the resulting ηTransfer, respectively. Considering the latter, we find that, even for our most heavily Fe2+ -doped sample (NFe = 7.5 × 1018 cm−3), Fedorov’s calculations suggest a value of ηTransfer < 0.3% (see Fig. 5c), i.e., a discrepancy of two orders of magnitude that mandates further clarification. To this end, we see two different approaches to resolving this discrepancy. On the one hand, all discussion so far has assumed a homogeneous distribution of ions in the ZnSe host, i.e., all Zn sites are populated by Cr2+ and Fe2+ with the same probability. An inhomogeneous ion distribution with clustered interionic distances would result in higher rates of excitation transfer. On the other hand, enhancement of the transfer rates could also arise from phonon-assisted processes. Future work is required to resolve this remaining open question.
Conclusions
For our study of the transfer efficiency, both singly-doped crystals and six co-doped ZnSe:Cr2+Fe2+ crystals were studied using time-resolved PL spectroscopy. In addition to the kinetics, great emphasis was placed on measuring and comparing the intensities of both PLs, from Cr2+ and Fe2+. The kinetics of the Cr2+ and Fe2+ emissions in the different crystals show a consistent pattern. In particular, when we resonantly excite the co-doped samples in the Cr2+ absorption band, we find clear indications for an efficient excitation transfer from Cr2+ to Fe2+, including a rapid initial rise time (~ 60 ns) of the transient, a delayed peak of about 200 ns after the peak of the excitation laser, and finally a significantly prolonged PL decay time of the Fe2+ PL of ~ 1 µs. Given the short 380-ns lifetimes of the Fe2+ ions, the latter finding can only be explained by replenishment via the Cr2+ → Fe2+ transfer process. Careful analysis of the PL measurements revealed a surprising finding, i.e., with efficiencies above 80%, the observed Cr2+ → Fe2+ transfer is much more efficient than theoretically expected. Accounting for non-radiative losses of Fe2+, even the overall efficiency is still an order of magnitude higher than estimated by Fedorov et al.3 Finally, mitigation of excessive non-radiative losses of the Fe2+ ions may be addressed by carefully adapted cooling of the co-doped laser crystals. If this remaining challenge can be solved, we are very optimistic about the Cr2+ → Fe2+ transfer, enabling a useful pumping scheme for short-pulse mid-infrared laser sources.
Availability of Data and Material
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
References
E. Sorokin, S. Naumov, and I.T. Sorokina, Ultrabroadband infrared solid-state lasers. IEEE J. Sel. Top. Quantum Electron. 11, 690 (2005).
T. Irina, Sorokina and Evgeni Sorokin, femtosecond Cr2+-based lasers. IEEE J. Select. Top. Quantum Electron. 21, 1601519 (2015).
V. Fedorov, T. Carlson, and S. Mirov, Energy transfer in iron-chromium co-doped ZnSe middle-infrared laser crystals. Opt. Mater. Express 9, 2340 (2019).
V.A. Antonov, K.N. Firsov, E.M. Gavrishchuk, V.B. Ikonnikov, I.G. Kononov, T.V. Kotereva, S.V. Kurashkin, S.V. Podlesnykh, S.A. Rodin, D.V. Savin, A.A. Sirotkin, A.M. Titirenko, and N.V. Zhavoronkov, Luminescent and lasing characteristics of polycrystalline Cr:Fe:ZnSe exited at 2.09 and 2.94 µm wavelengths. Laser Phys. Lett. 16(9), 095002 (2019). https://doi.org/10.1088/1612-202X/ab3851.
J. Peppers, V.V. Fedorov, and S.B. Mirov, Mid-IR photoluminescence of Fe2+ and Cr2+ ions in ZnSe crystal under excitation in charge transfer bands. Opt. Express 23, 4406 (2015).
T. Carlson, O. Gafarov, V. Fedorov, and S. Mirov, Presented at the Laser Congress 2018 (ASSL) (Boston: Massachusetts, 2018). ((unpublished)).
X.Y. Wang, Z. Chen, L. Zhang, B. Jiang, Xu. Min, J. Hong, Y. Wang, P. Zhang, L. Zhang, and Y. Hang, Preparation, spectroscopic characterization and energy transfer investigation of iron-chromium diffusion co-doped ZnSe for mid-IR laser applications. Opt. Mater. 54, 234 (2016).
P. Fürtjes, J.W. Tomm, U. Griebner, G. Steinmeyer, S.S. Balabanov, E.M. Gavrishchuk, and T. Elsaesser, Kinetics of excitation transfer from Cr2+ to Fe2+ ions in co-doped ZnSe. Opt. Lett. 47, 2129 (2022).
https://www.ipgphotonics.com/en/products/components/mid-ir-crystals
J.W. Evans, B.D. Dolasinski, T.R. Harris, J.W. Cleary, and P.A. Berry, Demonstration and power scaling of an Fe:CdMnTe laser at 52 microns. Opt. Mater. Express 7(3), 860 (2017). https://doi.org/10.1364/OME.7.000860.
S.B. Mirov, V.V. Fedorov, D. Martyshkin, I.S. Moskalev, M. Mirov, and S. Vasilyev, Progress in mid-IR lasers based on Cr and Fe-doped II–VI chalcogenides. IEEE J. Select. Top. Quantum Electron. 21(1), 292–310 (2015). https://doi.org/10.1109/JSTQE.2014.2346512.
P. Fuertjes, L. von Grafenstein, D. Ueberschaer, C. Mei, U. Griebner, and T. Elsaesser, Compact OPCPA system seeded by a Cr:ZnS laser for generating tunable femtosecond pulses in the MWIR. Opt. Lett. 46, 1704 (2021).
L.D. Deloach, R.H. Page, G.D. Wilke, S.A. Payne, and W.F. Krupke, Transition metal-doped zinc chalcogenides: spectroscopy and laser demonstration of a new class of gain media. IEEE J. Quantum Electron. 32, 885 (1996).
J.J. Adams, C. Bibeau, R.H. Page, D.M. Krol, L.H. Furu, and S.A. Payne, 4.0–4.5-µm lasing of Fe:ZnSe below 180 K, a new mid-infrared laser material. Opt. Lett. 24, 1720 (1999).
V.V. Fedorov, S.B. Mirov, A. Gallian, D.V. Badikov, M.P. Frolov, Y.V. Korostelin, V.I. Kozlovsky, A.I. Landman, Y.P. Podmar’kov, V.A. Akimov, and A.A. Voronov, 3.77–5.05-μm tunable solid-state lasers based on Fe2+-doped ZnSe crystals operating at low and room temperatures. IEEE J. Quantum Electron. 42(9), 907–917 (2006). https://doi.org/10.1109/JQE.2006.880119.
NoSoung Myoung, V.V. Fedorov, S.B. Mirov, and L.E. Wenger, Temperature and concentration quenching of mid-IR photoluminescence in iron doped ZnSe and ZnS laser crystals. J. Lumin. 132, 600 (2012).
M.E. Doroshenko, H. Jelínková, M. Jelínek, D. Vyhlídal, J. Šulc, N.O. Kovalenko Šulc, and I.S. Terzin, Influence of the pumping wavelength on laser properties of Fe2+ ions in ZnSe crystal. Opt. Lett. 44(7), 1686 (2019). https://doi.org/10.1364/OL.44.001686.
M. Surma, M. Godlewski, and T.P. Surkova, Iron and chromium impurities in ZnSe as centers of nonradiative recombination. Phys. Rev. B 50, 8319 (1994).
A. Burger, K. Chattopadhyay, J.O. Ndap, X. Ma, S.H. Morgan, C.I. Rablau, C.H. Su, S. Feth, R.H. Page, K.I. Schaffers, and S.A. Payne, Preparation conditions of chromium doped ZnSe and their infrared luminescence properties. J. Cryst. Growth 225, 249 (2001).
M. Godlewski, M. Surma, V.Y. Ivanov, and T.P. Surkova, Mechanisms of radiative and nonradiative recombination in ZnSe: Cr and ZnSe:Fe. Low Temp. Phys. 30, 891 (2004).
I. Radevici, K. Sushkevich, G. Colibaba, V. Sirkeli, H. Huhtinen, N. Nedeoglo, D. Nedeoglo, and P. Paturi, Influence of chromium interaction with native and impurity defects on optical and luminescence properties of ZnSe: Cr crystals. J. Appl. Phys. 114, 203104 (2013).
G. Steinmeyer, J.W. Tomm, P. Fuertjes, U. Griebner, S.S. Balabanov, and T. Elsaesser. Efficient electronic excitation transfer via phonon-assisted dipole-dipole coupling in Fe2+:Cr2+:ZnSe. Phys. Rev. Appl. 19, 054043 (2023).
T. Förster, Experimentelle und theoretische Untersuchung des zwischenmolekularen Übergangs von Elektronenanregungsenergie. Z. Nat. A 4, 321 (1949).
D.L. Dexter, A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836 (1953).
B. Henderson and M. Imbusch, Optical Spectroscopy of Inorganic Solids (Oxford: Oxford Science Publications, 2006), p.445.
M.E. Doroshenko, H. Jelinkova, A. Riha, M. Jelinek, M. Nemec, N.O. Kovalenko, and A.S. Gerasimenko, Mid-IR (44 µm) Zn1−xMnxSe:Cr2+, Fe2+ (x = 0.3) laser pumped by 17 µm laser using Cr2+-Fe2+ energy transfer. Opt. Lett. 44, 2724 (2019).
R.H. Page, K.I. Schaffers, L.D. DeLoach, G.D. Wilke, F.D. Patel, J.B. Tassano, S.A. Payne, W.F. Krupke, K.T. Chen, and A. Burger, Cr2+-doped zinc chalcogenides as efficient, widely tunable mid-infrared lasers. IEEE J. Quantum Electron. 33, 609 (1997).
Acknowledgments
We appreciate helpful discussions with Stanislav Balabanov, Oleg Pronin, Nazar Kovalenko, and Daniel Többens. Technical support from Sandy Schwirzke-Schaaf and Janett Feickert is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work has received funding from the European Union's Horizon 2020 research and innovation program under Grant agreement No. 871124 Laserlab-Europe.
Author information
Authors and Affiliations
Contributions
JWT and TE designed the research. PF and JWT carried out the TRPL experiment. PF, UG and JWT constructed the setup. GS conducted data analysis. All authors contributed to the discussion of the results and the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the content of this article.
Consent to Participate
All authors are agreed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomm, J.W., Steinmeyer, G., Fürtjes, P. et al. Excitation Transfer from Cr2+ to Fe2+ Ions in Co-doped ZnSe as a Pumping Scheme for Infrared Solid-State Lasers. J. Electron. Mater. 52, 5166–5171 (2023). https://doi.org/10.1007/s11664-023-10496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10496-6