Abstract
By using the solid-state reaction approach, composite polycrystalline samples of (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 were created with varying amounts of Bi2Se3, (x = 5%, 10%, 15%, and 20%). The hexagonal crystal structure of the composite was revealed by x-ray diffraction (XRD) with a space group of R\(\overline{3 }\)m. The surface of the samples was seen to have secondary particles using a field emission scanning electronic microscope. Every sample displayed the typical semi-conducting behaviour across the entire temperature range. In the complex (Bi0.98In0.02)2Te2.7Se0.3, it was found that bismuth was coordinated with six selenium atoms and there were significant selenium vacancies. With an increase in bismuth selenide concentration, the dissolution pattern shifted to a substitutional pattern. A two fold decrease in electrical resistivity for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 composition was seen compared to (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3. The granular material was produced by sintering and scattering of potential barrier, a thermal process that increases the Seebeck coefficient. A 200% increase was observed in thermopower for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 compared to (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3 compound.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The best thermoelectric (TE) figure of merit is found in bismuth telluride and its alloys, which are preferred materials for solid-state cooling systems. Since thermoelectric coolers do not need a compressor and are durable, dependable, and silent, they can function without any moving parts or compressors.1
The best TE materials now available are semiconducting materials such as Bi2Te3, and Bi2Se3, which have the smallest dimensionless figure of merit (ZT) of around 1.2.2 Although these materials have been in use for more than 50 years, they continue to attract interest because it is possible to structurally alter them to increase their ZT values. For instance, Kanatzidis et al.3 created cesium intercalated bismuth telluride (CsBi4Te6) with ZT of 2.4 at room temperature for p-type Bi2Te3/Sb2Te3 superlattice thin film. Subramanian and coworkers reported the highest figure of merit ever seen for TE materials.4
The weak Te(1)-Te(1)/Se(1)-Se(2) links between the quintuples and the layered structure of Bi2Te3/Bi2Se3 are the reason for the straightforward cleavage in the a and b directions. The basal planes have higher electrical conductivity than that along the c axis and across the van der Waals gap.5 The thermal conductivity of the basal plane in the perpendicular direction is greater than that of polycrystal. The basal planes have roughly twice the thermal conductivity in the direction perpendicular to the crystal than that of the polycrystal orientation. The Seebeck coefficient, however, is nearly isotropic for both n- and p-type systems.6 Meroz et al. reported creating n-type Bi2Te2.4Se0.6 by combining hot pressing with a melt-spinning technique that can maintain some of the desired crystallographic orientation needed for electrical optimization. At a temperature of 320 K, a high ZT value of 1.07 was attained.7 The thermoelectric characteristics of the Bi2Te3−xSex alloys were improved by Cai et al. via melt-spinning and resistance-pressing sintering.8 The thermoelectric characteristics of bulk compounds containing Cu-doped In2S3 were improved by Chen et al..9 Bismuth telluride-based solid solutions with a configurable optimal temperature range were created by Zhang et al..10 As far as we aware, doping on Bi2Se3 and Bi2Te3 has been extensively researched, while doping of these compounds simultaneously and in composite form has received the least attention. The low-temperature thermoelectric characteristics of the samples (Bi1−xInx)2Te2.7Se0.3, (Bi1−xSnx)2Te2.7Se0.3, (Bi1−xInx)2Se2.7Se0.3, and (Bi1−x Inx)2Se2.7Te0.3 were examined in prior publications.11,12,13,14,15 The (Bi1−xInx)2S2.7Te0.3 crystal develops lattice defects caused by the bismuth selenide composite impurities and precipitants. By incorporating additional bismuth telluride into the matrix, bismuth clusters are produced. With increasing bismuth telluride concentration, it is thought that bismuth occupancy in interstitial locations becomes less favoured and the dissolution pattern shifts to a substitutional pattern. Along with these, we recently reported the thermoelectric properties of the (Bi1−xInx)2Se2.7Te0.3/x%Bi2Te3 system. It was observed that anti-structure defects have a considerable impact on the carrier concentration in the current study’s n-type (Bi1−xInx)2Te2.7Se0.3 system.16 Therefore, in continuation of the previous report, the structural, surface morphological, and thermoelectric characteristics of different composite material systems such as (Bi0.98In0.02)2Te2.7Se0.3 with varying concentrations of Bi2Se3 (5%, 10%, 15%, and 20%) are examined in the high-temperature range of 30°C to 450°C.
Synthesis and Characterization Technique
The conventional solid-state reaction method is used to prepare polycrystalline samples of (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 of wt 5%, 10%, 15% and 20%, respectively.
-
(a)
Synthesis of (Bi0.98In0.02)2Te2.7Se0.3
(Bi0.98In0.02)2Te2.7Se0.3 was synthesized using a solid-state reaction approach; the precursors of bismuth (99.99%), indium (99.9%), selenium (99.995%), and tellurium (99.99%) were combined in a stoichiometric ratio and mixed for 2 h while vigorously grinding in an agate mortar. Pelletization of the powder was carried out using a 5-ton compression force. The pellets were placed in a quartz ampoule and vacuum-sealed for 30 h at 480°C in order to increase the uniformity and purity of compounds. The grinding process was repeated for the sintered pellets. The pellets were again sintered for 15 h at 250°C.
-
(b)
Synthesis of Bi2Se3
In a stoichiometric ratio, precursors of bismuth (99.99%) and selenium (99.995%) were mixed and processed for 2 h in an agate mortar. Pellets were created using a hydraulic press with 5-ton compression. The pellets were sintered in a quartz tube with a 12 mm diameter at 420°C for 24 h under 1.3 × 10–7 kPa vacuum. The grinding process was repeated for 1 h with the sintered samples to achieve the proper homogeneity and purity in the compound. After pelletization, this powder was sintered for 12 h at 200°C.
-
(c)
Composition of (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 for (x = wt 5%, wt 10%, wt 15%, wt 20%)
To obtain the desired homogeneity of the mixture, the produced Bi2Se3 was added individually to (Bi0.98In0.02)2Te2.7Se0.3 in 5%, 10%, 15%, and 20% composition, respectively, and thoroughly crushed in an agate mortar for 2 h. To densify the grains, the samples were sealed and sintered for around 12 h. The 10 × 2 × 5 mm3 pellets underwent a variety of experimental characterizations.
-
(d)
Characterization of synthesized compounds
X-ray diffraction (XRD) was conducted using an x-ray diffractometer (Rigaku MiniFlex) and Cu Kα rays to confirm the purity, crystallinity, and phase formation of the compounds. Field emission scanning electron microscopy (FESEM) was performed using a JEOL JSM-7100F instrument at a magnification of 35 kX and a voltage of 15 kV. Energy-dispersive x-ray spectroscopy (EDS) was used to identify the sample composition.
In the temperature range of 30°C to 450°C, the steady-state DC-method was used to concurrently determine the temperature-dependent electrical resistivity and the Seebeck coefficient.
Results and Discussion
X-ray Diffraction
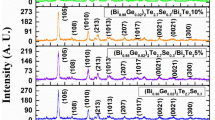
To identify the purity, crystalline phase, and crystallinity of the (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 composite, powder x-ray diffraction analysis was conducted between 20° and 80° at a scan rate of 2°/min. Figure 1a illustrates XRD plots for the (Bi1−xInx)2Te2.7Se0.3/Bi2Se3 system. All samples exhibit XRD patterns with a pronounced aligned XRD peak plane (015) and a hexagonal structure with space group R \(\overline{3 }\) m. The materials research project data sheet mp-568390 (Fig. 1b) is in good agreement with the current XRD patterns.17 Table I lists the characterization parameters including Rp, Rwp, Rep, and \(\chi \) 2 values. Significantly, as demonstrated in Fig. 4, the Te concentration significantly affects the XRD spectra. The Bi2Se3 composite decreases the changes in constants in a random way and causes the XRD peaks to migrate to higher angles because selenium has a smaller atomic radius than tellurium.18 Additionally, as the Se concentration reduces, the lattice constant derived from the XRD spectra drops. The position of the (015) peak, which is exclusively influenced by the lattice constant c (Fig. 2), shifts to a smaller angle and eventually to a higher angle on the 2θ side. The peak obtained at an angle of 30° from the pristine to the doped sample reveals a diminishing of the XRD peak, which indicates interlayer change due to the increase in the concentration of the doped compound. In the (Bi0.98In0.02)2Te2.7Se0.3 sample, Se is found to be deficient, indicating that there are several Se vacancies present in the lattice. (Bi0.98In0.02)2Te2.7Se0.3 has a layered lattice structure, and Te atomic layers are missing in the lattice. This results in an irregular variation in lattice constant of (Bi 0.98In0.02)2Te2.7Se0.3/Bi2Se3 (80%/20%) compared to other composite compounds. In addition, there is a change in lattice parameter c of (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 because of filling of Se in Te vacancies, resulting in a complicated layered structure. As evidence, the crystallite sizes are provided in Table I, which is in good agreement with lattice parameters.19,20 The discrepancy between theoretical and computed XRD peak patterns, which is depicted in Fig. 3, has been determined using EXPO 2014.21
Surface Morphological Features and Elemental Analysis
Images of (Bi0.98In0.02)2Te2.7Se0.3/BiSe3 composite samples captured with a field emission scanning electron microscope (FESEM) are shown in Fig. 4i. The (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 sample of 95%/5%, which has low porosity and a compact structure, is shown in Fig. 4i, a.18 It was found that the selenium particles were dispersed randomly across the surface of indium-doped Bi2Te3 with a rough surface (Fig. 4i, b). Secondary particles were often seen on the surface of the (Bi0.98In0.02)2Te2.7Se0.3/BiSe3 sample of 85%/15% by covering the tellurium or selenium atomic layer (Fig. 4i, c). The smooth surface of (Bi0.98In0.02)2Se2.7Te0.3/BiTe3 with 80%/20% (Fig. 4i, d) illustrates the standardized distribution of the composites in the grains.19,20
EDS was used to check whether the samples had any additional components, and the results are presented in Fig. 4. (ii) Bismuth (L line), selenium (K line), indium (K line), tellurium (K line), and selenium (L line) are present in all of the (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 samples. Additionally, EDS elemental mapping (Fig. 5) demonstrates that the elements were uniformly dispersed across the sample surface. The observed atomic percentage is approximately matching with the nominal percentage of composition (Table II).
Electrical Resistivity
The electrical resistivity of (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 composites was determined in the temperature range of 30–450°C (Fig. 6). All the samples exhibit n-type semiconducting behaviour in the entire temperature range. In the (Bi0.98In0.02)2Te2.7Se0.3complex, it was found that bismuth coordinated with six selenium atoms and there were significant selenium vacancies.22,23,24,25 The indium dopant in the compound disturbs this coordination. The places in the host lattice where indium and selenium are coordinated appear to have a higher volume than the host.26 All the samples exhibited the usual semiconducting behaviour because of the scattering of carriers in grain boundaries and the point defects established by the random distribution of Te and Se in the crystallite positions. The (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3 and (Bi0.98In0.02)2Te2.7Se0.3/10%Bi2Se3 samples are found to have higher electrical resistivity than (Bi0.98In0.02)2Te2.7Se0.3/15%Bi2Se3 due to the self-interacting percolation channels. This causes the impurity atoms to be spatially redistributed.27 Clusters of bismuth are generated by adding more bismuth selenide to the matrix. It is assumed that bismuth occupation in interstitial sites is less preferred with increasing bismuth selenide content and the dissolution pattern changes to a substitutional pattern. In addition, the substitution of 0.02 In for (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 decreased the electrical resistivity. This suggests that In acts as a scattering centre that disturbs electron conduction. Hence, there is a twofold decrease in electrical resistivity for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 composition relative to that of (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3.
In the (Bi1−xInx)2Te2.7Se0.3/x%Bi2Te3 system, at the high temperature zone, the hopping mechanism between nearest neighbouring sites is caused by thermally activated tiny polarons, as shown by the linear plot between ln(ρ/T) and 1/T in Fig. 7. Because selenium atom composition may change after sintering, EA values are reported to fluctuate inconsistently with the doping concentration.28,29 This causes the (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 samples to power scattering centres.30
Seebeck Coefficient and Thermopower
The Seebeck coefficient was determined in the temperature range of 30–450°C for the synthesized polycrystalline samples and is displayed in Fig. 8. When Te/Se sites are occupied by Bi and In atoms in n-type (Bi1−xIn)2Te2.7Se0.3/Bi2Se3 alloys, anti-structure defects are formed. It was observed that a decrease in the electronegativity disparity between the component atoms was conducive to an increase in the formation of anti-structure defects.31,32 Because of poor van der Waals connection between their layers, cleavage slip of mechanical deformation caused selenium vacancies to arise during the sintering process. The Bi atoms will enter Se vacancies because of the smaller difference of electronegativity between them. An anti-site defect was produced, resulting in the formation of the vacancies VBi.33 Therefore, the concentration of carriers increases in (Bi1−xInx)2Te2.7Se0.3/Bi2Se3 as the content of Bi2Te3 increases. At higher temperatures, thermal excitation of carriers reduces bipolar transport, which also shifts the temperature dependence of the Seebeck value. Additionally, potential barrier scattering, a thermally increases the Seebeck coefficient, in the highly granular material by sintering. As a result, there is a Gaussian-shaped curve of Seebeck coefficients at 350°C for all the samples.34 The theoretical carrier concentration is calculated using equation
where m* is the effective mass of the electron, e is the elementary charge of an electron, h is the Planck’s constant, n is the carrier concentration, and T is the absolute temperature, where S(T) is the temperature-dependent Seebeck coefficient, kB is Boltzmann’s constant.35 The mobility can be calculated using
The theoretical values of charge density, mobility, activation energy and scattering factors of (Bi1−xInx)2Te2.7Se0.3/Bi2Se3 were calculated and are given in Table III.
In addition, the temperature-dependent power factor of the investigated (Bi1−xInx)2Te2.7Se0.3/Bi2Se3 composites is shown in Fig. 9. The pristine (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3 has the lowest thermopower of 45 µW/mK2 at 300°C, but the greatest thermopower of nearly 100 µW/mK2 was found for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3. There is a 200% increase in thermopower for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 relative to (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3.
Conclusions
The thermoelectric characteristics of the (Bi0.98In0.02)2Te2.7Se0.3/x%Bi2Se3 composite system with composition of 5%, 10%, 15% and 20% are investigated in this paper. The hexagonal crystal structure with a space group of \(R\overline{3 }\) m is confirmed by the XRD study. When selenium or tellurium is coated in an atomic layer, secondary particles are observed under a field emission scanning electronic microscope on the sample surface. Every sample displayed semiconducting behaviour across the entire temperature range. The significant impact of grain boundaries on the electrical characteristics of the samples can be seen in the reported electrical resistivity, which results in a larger concentration of scattering centres in the composites. With an increase in bismuth selenide concentration, the bismuth occupancy in interstitial sites becomes less favoured, and the dissolution pattern switches to a substitutional pattern. Hence, there is a twofold decrease in electrical resistivity for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 composition compared to (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3. Potential barrier scattering is a thermal process that raises the Seebeck coefficient of a material because of sintering. The Seebeck coefficients have a Gaussian form at 350°C for all the samples. There is a twofold increase in thermopower for (Bi0.98In0.02)2Te2.7Se0.3/20%Bi2Se3 relative to (Bi0.98In0.02)2Te2.7Se0.3/5%Bi2Se3.
Data Availability
The thermoelectric data were generated at the Centre for Clean Energy, Department of Physics, Manipal Institute of Technology. The derived data supporting the findings of this study are available from the corresponding author [ANP] on request.
References
K. Kim, G. Kim, H. Lee, K.H. Lee, and W. Lee, Band engineering and tuning thermoelectric transport properties of p-type Bi0.52Sb1.48Te3 by Pb doping for low-temperature power generation. Scr. Mater. 145, 41 (2018).
Z. Ali, S. Butt, C. Cao, F.K. Butt, M. Tahir, M. Tanveer, I. Aslam, M. Rizwan, F. Idrees, and S. Khalid, Thermochemically evolved nanoplatelets of bismuth selenide with enhanced thermoelectric figure of merit. AIP Adv. 4(11), 117129 (2014).
D.-Y. Chung, T.P. Hogan, M. Rocci-Lane, P. Brazis, J.R. Ireland, C.R. Kannewurf, M. Bastea, C. Uher, and M.G. Kanatzidis, A new thermoelectric material: CsBi4Te6. J. Am. Chem. Soc. 126(20), 6414 (2004).
H. Böttner, G. Chen, and R. Venkatasubramanian, Aspects of thin-film superlattice thermoelectric materials, devices, and applications. MRS Bull. 31(3), 211 (2006).
J. Horák, J. Navrátil, and Z. Starý, Lattice point defects and free-carrier concentration in Bi2+xTe3 and Bi2+xSe3 crystals. J. Phys. Chem. Solids 53(8), 1067 (1992).
S.V. Ovsyannikov, V.V. Shchennikov, G.V. Vorontsov, A.Y. Manakov, A.Y. Likhacheva, and V.A. Kulbachinskii, Giant improvement of thermoelectric power factor of Bi2Te3 under pressure. J. Appl. Phys. 104(5), 053713 (2008).
O. Meroz, N. Elkabets, and Y. Gelbstein, Enhanced thermoelectric properties of n-type Bi2Te3–xSex alloys following melt-spinning. ACS Appl. Energy Mater. 3, 2090 (2020).
X. Cai, X. Fan, Z. Rong, F. Yang, Z. Gan, and G. Li, Improved thermoelectric properties of Bi2Te3−xSex alloys by melt spinning and resistance pressing sintering. J. Phys. D. Appl. Phys. 47, 115101 (2014).
Y.X. Chen, F. Li, W. Wang, Z. Zheng, J. Luo, P. Fan, and T. Takeuchi, Optimization of thermoelectric properties achieved in Cu doped β-In2S3 bulks. J Alloys Compd. 782, 641 (2019).
Zhai, Y. Wu, T.-J. Zhu, and X.-B. Zhao, Tunable optimum temperature range of high-performance zone melted bismuth-telluride-based solid solutions. Cryst. Growth Des. 18, 4646 (2018).
G.S. Hegde, A.N. Prabhu, A. Rao, and P.D. Babu, Enhancement of thermoelectric performance of In doped Bi2Te2.7Se0.3 compounds. Phys. B Condens. Matter 584, 412087 (2020).
G.S. Hegde, A.N. Prabhu, Y.H. Gao, Y.K. Kuo, and V.R. Reddy, Potential thermoelectric materials of indium and tellurium co-doped bismuth selenide single crystals grown by melt growth technique. J. Alloys Compd. 866, 158814 (2021).
G.S. Hegde, A.N. Prabhu, R.Y. Huang, and Y.K. Kuo, Reduction in thermal conductivity and electrical resistivity of indium and tellurium co-doped bismuth selenide thermoelectric system. J. Mater. Sci. Mater. Electron. 31(22), 19511 (2020).
G.S. Hegde, A.N. Prabhu, and M.K. Chattopadhyay, Improved electrical conductivity and power factor in Sn and Se co-doped melt-grown Bi2Te3 single crystal. J. Mater. Sci. Mater. Electron. 32(20), 24871 (2021).
G.S. Hegde, A.N. Prabhu, A. Rao, and M.K. Chattopadhyay, Enhancement in thermoelectric figure of merit of bismuth telluride system due to tin and selenium co-doping. Mater. Sci. Semicond. Process. 127, 105645 (2021).
G.S. Hegde, A.N. Prabhu, A. Rao, K. Gurukrishna, and U. Deepika Shanubhogue, Investigation of near-room and high-temperature thermoelectric properties of (Bi0.98In0.02)2Se2.7Te0.3/Bi2Te3 composite system. J. Mater. Sci. Mater. Electron. 33, 25163 (2022).
H. Fang, J.-H. Bahk, T. Feng, Z. Cheng, A.M.S. Mohammed, X. Wang, X. Ruan, A. Shakouri, and Y. Wu, Thermoelectric properties of solution-synthesized n-type Bi2Te3 nanocomposites modulated by Se: an experimental and theoretical study. Nano Res. 9(1), 117 (2016).
A. Altomare, C. Cuocci, C. Giacovazzo, A. Moliterni, R. Rizzi, N. Corriero, and A. Falcicchio, EXPO2013: a kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 46(4), 1231 (2013).
N.K. Singh, J. Pandey, S. Acharya, and A. Soni, Charge carriers modulation and thermoelectric performance of intrinsically p-type Bi2Te3 by Ge doping. J. Alloys Compd. 746, 350 (2018).
S. Byun, J. Cha, C. Zhou, Y.K. Lee, H. Lee, S.H. Park, W.B. Lee, and I. Chung, Unusual n-type thermoelectric properties of Bi2Te3 doped with divalent alkali earth metals. J. Solid State Chem. 269, 396 (2019).
S. Chen, K.F. Cai, F.Y. Li, and S.Z. Shen, The effect of Cu addition on the system stability and thermoelectric properties of Bi2Te3. J. Electron. Mater. 43(6), 1966 (2014).
Y. Xiao, J. Yang, G. Li, M. Liu, L. Fu, Y. Luo, W. Li, and J. Peng, Enhanced thermoelectric and mechanical performance of polycrystalline p-type Bi0.5Sb1.5Te3 by a traditional physical metallurgical strategy. Intermetallics 50, 20 (2014).
O. Caha, A. Dubroka, J. Humlíček, V. Holý, H. Steiner, M. Ul-Hassan, J. Sánchez-Barriga, O. Rader, T.N. Stanislavchuk, A.A. Sirenko, G. Bauer, and G. Springholz, Growth, structure, and electronic properties of epitaxial bismuth telluride topological insulator films on BaF2 (111) substrates. Cryst. Growth Des. 13(8), 3365 (2013).
R. Suriakarthick, M. Senthil Pandian, P. Ramasamy, R. Kumar Raji, M. Muralidharan, C. Amaljith, and S. Sagadevan, Solvothermal synthesis, structural and transport properties of polycrystalline copper tin selenide for thermoelectric applications. Inorg. Chem. Commun. 140, 109491 (2022).
M.-K. Han, J. Hwang, and S.-J. Kim, Improved thermoelectric properties of n-type Bi2Te3 alloy deriving from two-phased heterostructure by the reduction of CuI with Sn. J. Mater. Sci. Mater. Electron. 30(2), 1282 (2019).
L.A. Kuznetsova, V.L. Kuznetsov, and D.M. Rowe, Thermoelectric properties and crystal structure of ternary compounds in the Ge(Sn, Pb)Te-Bi2Te3 systems. J. Phys. Chem. Solids 61(8), 1269 (2000).
X.S. Zhou, Y. Deng, C.W. Nan, and Y.H. Lin, Transport properties of SnTe-Bi2Te3 alloys. J. Alloys Compd. 352(1–2), 328 (2003).
A. Banerjee, S. Pal, and B.K. Chaudhuri, Nature of small-polaron hopping conduction and the effect of Cr doping on the transport properties of rare-earth manganite La0.5Pb0.5Mn1−xCrxO3 nature of small-polaron hopping conduction and the effect of Cr doping. J. Chem. Phys. 115, 1550 (2001).
I. Ahmad, M.J. Akhtar, M. Younas, M. Siddique, and M.M. Hasan, Small polaronic hole hopping mechanism and Maxwell–Wagner relaxation in NdFeO3. J. Appl. Phys. 112(7), 074105 (2012).
J.U. Rahman, N. Van Du, W.H. Nam, W.H. Shin, K.H. Lee, W.S. Seo, M.H. Kim, and S. Lee, Grain boundary interfaces controlled by reduced graphene oxide in nonstoichiometric SrTiO3-δ thermoelectrics. Sci. Rep. 9(1), 1 (2019).
S. Lin, W. Li, Z. Chen, J. Shen, B. Ge, and Y. Pei, Tellurium as a high-performance elemental thermoelectric. Nat. Commun. 7(1), 10287 (2016).
Z.-H. Ge, Y.-H. Ji, Y. Qiu, X. Chong, J. Feng, and J. He, Enhanced thermoelectric properties of bismuth telluride bulk achieved by telluride-spilling during the spark plasma sintering process. Scr. Mater. 143, 90 (2018).
L.D. Zhao, B.-P. Zhang, J.-F. Li, H.L. Zhang, and W.S. Liu, Enhanced thermoelectric and mechanical properties in textured n-type Bi2Te3 prepared by spark plasma sintering. Solid State Sci. 10(5), 651 (2008).
M. Gharsallah, F. Serrano-Sanchez, N.M. Nemes, J.L. Martinez, and J.A. Alonso, Influence of doping and nanostructuration on n-type Bi2(Te0.8Se0.2)3 alloys synthesized by Arc melting. Nanoscale Res. Lett. 12(1), 47 (2017).
K.S. Prasad, A. Rao, B. Gahtori, S. Bathula, A. Dhar, C.-C. Chang, and Y.-K. Kuo, Low-temperature thermoelectric properties of Pb doped Cu2SnSe3. Phys. B Condens. Matter 520, 7 (2017).
Acknowledgments
Ganesh Shridhar Hegde (GSH) would like to thank the Manipal Academy of Higher Education for helping to support Dr. T. M. A.'s PhD scholarship. ANP thanks UGC-DAE, Mumbai, for providing financial support in accordance with the provisions of the project grant UDCSR/MUM/AO/CRS-M-314/2021/298. One of the authors (AR) received the money required to finish this investigation from the DST-FIST Grant (SR/FIST/PS-1/2017/8).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The research leading to these results received funding from project grant UDCSR/MUM/AO/CRS-M-314/2021/298, Dr. T. M. A.’s PhD scholarship and DST-FIST Grant (SR/FIST/PS-1/2017/8).
Author information
Authors and Affiliations
Contributions
GSH: Methodology, Writing—original draft, Formal analysis. SP: Calculation and Plotting of Graphs. ANP: Supervision, Validation. AR: Writing—review & editing. KG and DSU: Data curation.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hegde, G.S., Prabhu, A.N., Putran, S. et al. Thermoelectric Composite of (Bi0.98In0.02)2Te2.7Se0.3/Bi2Se3 with Enhanced Thermopower and Reduced Electrical Resistivity. J. Electron. Mater. 52, 3749–3758 (2023). https://doi.org/10.1007/s11664-023-10346-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10346-5