Abstract
An oxyflouroborate glass series of composition 75B2O3-5Al2O3–(20-x) Li2O–xMgF2 (where x = 0, 5, 10, and 15 mol.%) was prepared using the normal melt-quenching technique. The physical properties (density, molar volume, and different optical behaviors) of the glass system were investigated via different techniques and discussed with the substitution of Li2O for MgF2. Also, the absorption coefficient, both direct and indirect optical energy gaps, and the optical exciton energy gap were studied. Furthermore, IR spectroscopy was used as a structural probe of the nearest-neighbor environment in the glass network. The results elucidate that the replacement of Li2O with MgF2 leads to both a blueshift in absorption cutoff and a decrease in the direct energy gap. More liberation of Mg+2 ions produces more localized states during transition, which decreases the values of band gap energy. The addition of MgF2 has a clear impact on lowering the glass phonon energy, which makes this glass promising for fiber amplifiers that operate at certain telecommunications wavelength bands and for upconversion fiber lasers. These results show the capability of using oxyflouroborate glass series to be applicable in optical amplifier laser components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the development of photonic materials has become more significant for the progress of photonics applications and the telecommunications industry.1,2,3 Upconversion is the mechanism that converts low-energy photons into visible light.4 The most effective upconverters must consist of host systems that provide low non-radiative losses so that radiative emission (luminescence) could be enhanced. Different glass hosts such as borate, phosphate, germanite, vanadate, and tellurite families have been extensively studied for this objective.5 Among these families, borate glasses are preferred in certain optical, optoelectronic and photonic devices mainly owing to their (i) low working temperatures, (ii) wide glass forming range, (iii) second- and third-order optical non-linearity6 and borate glasses are most consistent for high concentrations of transition metal and rare earth ion doping.7 The borate glasses modified by various constituents such as alkali oxide, alkaline-earth oxide, aluminum oxide, fluorides, and heavy metal oxides have the advantages of high transparency, high thermal stability, low phonon energy compared to that of pure borate glass, which is high (1400 cm−1).8,9 The oxyfluoride borate glasses are one of the highly efficient upconversion luminescence hosts; they not only have higher chemical and mechanical stability but also lower phonon energy.10 The modifier oxides influence the high covalent strength B-O bonds, creating the so-called borate anomaly (the existence of different anionic tri-coordinate borate and four-coordinate borate species). The increase of the disorder degree of the glass structure results in a high density of localized states in the allowed energy band tails. This gives the glass its optoelectronic properties.11 Since the photonic property is related to the ionic character of the glass network, it is necessary to tune the structure of glass to enhance the conversion of covalent to ionic conditions to adjust the energy band gap of glass. Accordingly, the effect of MgF2 on lithium alumino-borate glass is studied for upconversion applications.
In the field of developing the glass structure system by embedding rare earth elements or transition metals for enhancing their phonon and optical characteristics to be used in optoelectronic applications, In 2013, Zhou et al. studied Er3+-doped fluorotellurite glasses to be applied in the development field of optical laser amplifier components as this structure leads to emission of infrared radiation (1.32 µm) due to the phonon characteristic of the fluorotellurite glass structure.12 In 2018, Qi et al. succeeded in preparing a new series of fluorochloride glasses based on the structure of fluoroaluminate with varying amounts of Cl−, which showed a novel phonon characteristic. This perspective was discussed as the increase of chlorine ions in this kind of glass structure leads to an increase in the thermal stability of their host structure, which allows them to be applied in aser materials.13 In 2019, Marzouk et al. studied the effect of doping MnO2 and MoO2 on two different glass matrixes from sodium phosphate glasses, which affected the UV and absorption characteristics, followed by developing of its optical energy band gap values due to formation of modifier groups from manganese ions, molybdenum ions, and phosphate ions.14 In 2020, Al-Mokhtar et al. found that the addition of MgF2 to some borate glass increases non-bridging oxygen, followed by increasing the disorder of its system, which affected the development of its optical properties by generation of a transmission band at the short wavelength side and an absorption edge at the long wavelength side; consequently, as the embedding of MgF2 in the glass matrix increases, its energy ΔE increases.15 In 2021, El-Daly et al. synthesized and investigated the optical characteristics of cobalt borate glass, which showed an increase in phonon characteristics as the amount of embedded Co increased due to the interatomic spacing effect in the prepared glass structure.16
In this work, an oxy-fluoro borate glass series of composition 75B2O3-5Al2O3–(20-x) Li2O–xMgF2 (where x = 0, 5, 10, and 15 mol.%) was prepared using the normal melt-quenching technique to study the change in their optical characteristics. The effect of MgF2 on the optical features of the main glass structure was investigated by transition and vibration absorption spectroscopy. This study was carried out to test the capability for using the prepared glass system material in the development field for optical laser amplifier or optical fiber
Methodology
Preparation of Glass Samples
The chemical composition of 75B2O3-5Al2O3-(20-x) Li2O-xMgF2 (where x = 0, 5, 10, and 15 mol.%) is designed to prepare a series of glass samples by melt-quenching. The starting materials are reagent grade chemicals of H3BO3, Al2O3, Li2CO3 and MgF2. Certain amounts of these chemicals are mixed well and melted at 1000°C in a porcelain crucible for 1 h in an electric glow-bar furnace. Continuous stirring was done during the melting to ensure complete homogeneity. The melts are then poured rapidly into a stainless-steel mould of defined dimensions and annealed in a preheated furnace at a temperature of 400°C and left to cool gradually to room temperature at a rate of 30°C/h to relieve any internal mechanical stresses. The shaped samples are polished well to measure their physical and optical properties.
Characterisation
The FTIR spectra of the studied glasses were recorded by a computerized FTIR spectrometer (type Bruker VERTEX 8V, Germany) covering the wavenumber range of 400–4000 cm−1 with a resolution of 4 cm−1. Spectroscopic measurements: A UV/VIS/NIR spectrophotometer (Jasco V-770, Japan) was used to collect the optical absorption and reflectance spectra for the samples at ambient conditions. The density of glass samples (\(\rho glass\) ) is measured by the Archimedes rule, and xylene is the immersion liquid, using the next formula,
where Wa is the weight of samples in air while Wx is the weight of samples in liquid. The molar volume Vm of glasses was calculated by using Eq. (2):
where xi is the molar fraction and Mi is the molecular weight of the ith component of the studied compositions.
Results and Discussion
Density and Molar Volume Calculation
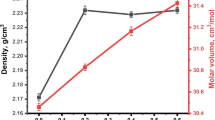
Firstly, supplementary Figure S1 depicts the X-ray diffraction patterns of a series of MgF2-containing glasses. The findings show that all of the synthesized glasses are amorphous, with no hint of crystalline phases. The density (glass) and molar volume (Vm) values of the 75B2O3–5Al2O3–(20-x)Li2O–xMgF2 glass samples with increasing substitution amounts of MgF2 are displayed in Fig. 1. The measured values of density show an anomalous behavior, as depicted in this figure. As boosting the glass network with 5 mol.% of MgF2 at the expense of Li2O increased the density value, a decrease to a minimum density value versus the increase of the MgF2 ratio to 10 mol.% is observed. This anomalous behavior is followed by a new increase in density value as the molar fraction, x, rises to its maximum value at 15 mol.%. However, enhancing the glass network with substituting amounts of MgF2 gradually increased the molar volume values. Molar volume is more sensitive to structural differences between glasses than density because it normalizes for atomic weights of different glass components. The observed increase in VM demonstrates that the packing of the coordination polyhedral in the structural glass matrix of the alumino-fluoroborate glasses improves with increasing MgF2 content.17
The anomalous behavior of density can be explained as follows. The structure of the present glass network depends mainly on the two construction units of the borate network, BO30 (normal triangle coordination) and BO4− (fourfold tetrahedral coordination) that are linked together via oxygen atoms to create B-O-B bonds. Firstly, the modifying action of MgF2 converts part of BO30 into BO4−. This conversion process increases the amount of bridging oxygen (BO) and consequently increases the connectivity and compactness of the studied glass network.18 Moreover, the addition of MgF2 breaks up the local symmetry of the structural BO4− units and converts them into neutral structural units BO30.18 Therefore, MgF2 can form –O–Mg–O and Mg–O–B bonds, creating an open structure with a greater number of BO30 units than BO4−. This conversion creates coordinated non-bridging oxygen (NBO) (oxygen dangling bond) defects. The maximum addition of MgF2 modifier to the studied borate network provides the network with excess Mg ions. The big difference between the atomic weights and densities of the modifier Mg+2 is (At. W = 24.305 u, ρ = 1.738 g/cm3) and Li+ is (At. W = 6.941 u, ρ = 0.534 g/cm3). Hence, Mg+2 ions are more efficient at filling the interstitial spaces in the glass network structure than Li+ ions, leading to the formation of a more compact network.
FTIR Spectroscopic Measurements
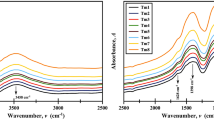
Figure 2 illustrates the structural FTIR vibrational bands of the studied glasses. It is clear that there are four band regions. The first is in the far infrared range of about 400–500 cm−1, and the second is cited at about 700cm−1. The two other bands are broad and extend from 800–1200 cm−1 and 1200–1400 cm−1 , respectively. By increasing MgF2 content, unremarkable shifting for the bands is cited in the near IR region, while the band at about 700 cm−1 remains as it is. The strong changes are remarkable at the two broad band regions 800–1200 cm−1 and 1200-1400 cm−1, where more broadness is detected at 800–1200 cm−1 and increasing intensities at bands observed at 1200–1400 cm−1. More data can be observed in Fig. 3, where the deconvoluted graphs split the broad band into several sharp bands. These sharp bands are listed in Table I.
From all three IR figures, it can be recognized that the bands cited at 424 and 472 cm−1 are related to the vibration of Li+ ions, while the band at 461 cm−1 which formed after the addition of MgF2 is due to the vibrations of Mg2+ cations in their sites. A bending vibrational absorption band is centered at about 690 cm−1, which is correlated to B–O–B.19 Three bands observed at about 806, 920 and 1063 cm−1 are related to the stretching vibrations of BO4 tetrahedral borate groups.20 After replacing Li2O by MgF2 , these three bands were shifted to about 901, 1011, and 1099 cm-1 , respectively. These band changes can be interpreted as the formation of MgO4 groups instead of BO4.21 Finally, the bands at about 1227, 1360 and 1473 cm−1 , which are related to the symmetric and asymmetric stretching vibrations of boroxol groups BO3, are shifted to 1227, 1435 and 1506 cm−1, and the formation of a new absorption band at about 1344 cm−1 is caused by the increase of Mg2+ ions in the glass system.21
Sub-Band Gap Structural Properties
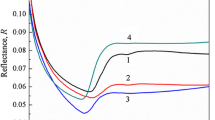
The study of the transmittance and reflectance of the samples with different composite structures as a function of different wavelengths is shown in Fig. 4a,b. From Fig. 4a, the glass transmittance of the main structure of 75B2O3.(20-x)Li2O.5Al2O3.xMgF2 was increased from 53% to 77%, 65%, and 67% for the addition of 5, 10, and 15 mol.% of MgF2, respectively. This significant change in the transmittance value is related to the incorporation of MgF2 in different amounts. The amount of addition of MgF2 was affected to increase the transmittance optical properties of the main glass structure of 75B2O3.20Li2O.5Al2O3 to 24%, 12%, and 14% when the molar amounts were changed to be 5, 10, and 15 mol.%. According to the traditional polarization theory related to the transmittance spectral line, the increase in the transmission spectral line may be related to the addition of the MgF2 structure instead of Li2O with its characteristic broad energy band gap and high bonding strength. Also, each spectral lline is responsible for an individual characteristic absorption cutoff wavelength as the main glass structure with any molar percentage addition of MgF2 has a 371-nm absorption cutoff wavelength. This value showed a blueshift to a higher wavelength after the addition of different molar percentages of MgF2 were 426, 410, and 415 for the addition of 5, 10, and 15 mol.% of MgF2, respectively. This change is related to the change in the molecular weight of the main glass structure under the effect of embedding of MgF2 instead of the number of molecular structures from Li2O.
From the transmittance (T) and reflectance (R) of the different prepared structural forms of the main glass structure, the absorbance coefficient of the glass (\(\alpha \)) can be calculated from the Beer-Lambert law:
where L is the thickness of the glass sample. The absorbance and transmittance investigations depend on changes in the energy band gap values and type of transition. The value of the energy band gap and the type of transition (direct or indirect) can be detected based on the Tauc relation.
where α is the absorption coefficient, hυ is the discrete photon energy, B is a constant, n is the type of transition (2 in the case of direct transition or ½ in the case of indirect transition), Eg = \(h\upsilon \) is the optical energy band gap, and \(\upsilon \) is the frequency. By plotting the relation between \({(\alpha h\upsilon )}^{2}\) versus Eg, the detection of the energy band gap from the direct transition was detected. It showed that the energy band gap of the direct transition for the main glass structure after its embedding is 4.13 eV, while this value was changed to be 3.67, 3.91, and 3.87 eV for the addition of 5, 10, and 15 mol.% of MgF2, respectively, as shown in Fig. 4c. In other words, the relationship between \({(\alpha h\upsilon )}^{0.5}\) versus \(h\upsilon \) represents the detection of energy band from an indirect transition. It is clear that the energy band gap for the main glass structure after its embedding is 1.64 eV, while this value was changed to be 2.69, 2.31, and 2.58 eV for the addition of 5, 10, and 15 mol.% of MgF2, respectively, as shown in Fig. 4d. From Fig. 4c–d, it is clear that there is a variation between the energy band gap in the case of direct transition in compared with indirect transition. In addition, the variation of the energy band gap has the same type of transition when the molar ratio of the main glass structure was changed, which is related to the embedding process of the main glass structure by decreasing the molar amount of Li2O and increasing the molar amount of MgF2 leading to the enhancement of the polymerization process, which allowed the appearance of more localized states during transition affected by the values of the energy band gap.22,23 So it was concluded that the energy band gap increased with increased embedding of MgF2 in the matrix glass, which is related to the transition produced from the HOMO levels and LUMO levels in their crystal structure. When the fluoroborate glass structure was transferred to alkali borate and alkali fluoroborate glass structure, BO4 units with excess amounts were generated. The LUMO level of these alkali structures consisted of vacant orbitals caused by oxygen atoms of borate oxide structure and the energy band gap of pure borate oxide structure is around 8 eV. When the MgF2 structure started to be embedded, the HOMO level started to be reduced, and consequently, caused a decrease of the energy band gap value (as mentioned for 5 mol.% of MgF2), and was followed by increasing the energy band gap value as the amount of fluorine increased (as mentioned for 10 mol.% of MgF2) due to the effect embedding of fluorine. After that, the energy band gap started to decrease again as the amount of fluorine further increased (as mentioned for 15 mol.% of MgF2) due to the creation of non-bridging Mg, which bound excited electrons less firmly as the bridging Mg increases with MgF2, leading to a reduction in the energy band gap value.15,24
Furthermore, the previously calculated energy band gap appearing in Fig. 4c–d could be represented by the exciton energy band gap (\({E}_{\mathrm{gap}-\mathrm{ext}}\)), which could be used to inform the optical energy band gap (\({E}_{\mathrm{gap}-\mathrm{opt}}\)) of the glass structure before and after the addition of MgF2 by studying the 1st derivative of the optical absorbance values of the different glass structures as a function of photon energy values as shown in Fig. 5a. The maximum detection peak in each spectrum could be represented by the optical energy band gap (\({E}_{\mathrm{gap}-\mathrm{opt}}\)). The changes of the optical energy band gap compared with the exciton energy band gap could be confirmed from Fig. 5b.25,26 of the different glass structures with different molar percentages of MgF2
According to the network structure of the glass formation, the optical transition was changed, which affected the energy band gap transition either directly or indirectly. The behavior of the changes in the optical frequencies molecular of the main glass structure was changed as the amount of MgF2 was embedded, which is represented by phonon energy. The phonon energy (EΩ) could be calculated based on the following formula:
where \({{E}_{\mathrm{opt}}}^{d}\) and \({{E}_{\mathrm{opt}}}^{\mathrm{ind}}\) are the energy band gap from direct transition and indirect transition of the glass structure of 75B2O3.(20-x)Li2O.5Al2O3.xMgF2 before and after the addition of MgF2 with different molar percentages of MgF2, respectively. The factor 2 in the phonon energy calculation is related to the presence of two electrons in each momentum-energy. From this calculation, the phonon energy of the glass structure of 75B2O3.(20-x)Li2O.5Al2O3.xMgF2 before and after the addition of MgF2 with different molar percentages of MgF2 as a function of the molar ratio of MgF2/(LiO2 + MgF2) was studied as shown in Fig. 6. From this figure, it is clear that the glass structure has low phonon energy, which allows it to be applied in fiber amplifiers.11,27,28
Conclusion
A novel oxyflouroborate glass is prepared with the replacement of Li2O by MgF2 intentionally to reduce the glass phonon energy. An increase in glass molar volume (VM) demonstrates that the packing of the coordination polyhedra in the structural glass matrix of the studied glasses improves with increasing MgF2 with liberation of Mg+2 ions that are more able to fill the interstitial spaces in the glass network structure than Li+ ions leading to the formation of more compact network. FTIR deconvoluted spectra confirmed the role of Mg+2 on the structural network with successive increases of MgF2 until 15 mol.%. Moreover, direct, indirect, and exciton optical energy band gaps are differently affected with the implanted MgF2, while the glass phonon energy is decreased from 1.2 eV to 0.6 eV, which makes the designed new system promising for fiber amplifiers that operate at certain telecommunications wavelength bands and for upconversion fiber lasers.
References
J.S. Kumar, A.M. Babu, T. Sasikala, and L.R. Moorthy, NIR Fluorescence and Visible Upconversion Studies of Nd3+ Ions in Calcium Fluoroborate Glasses. Chem. Phys. Lett. 484, 207 (2010).
M. Abdel-Baki, and F. El-Diasty, Glasses for Photonic Technologies. Int. J. Opt. Appl 3, 125 (2013).
B. Karthikeyan, R. Philip, and S. Mohan, Optical and Non-linear Optical Properties of Nd3+-Doped Heavy Metal Borate Glasses. Opt. Commun. 246, 153 (2005).
C.B. de Araújo, G.S. Maciel, L.S. de Menezes, N. Rakov, E.L. Falcão-Filho, V.A. Jerez, and Y. Messaddeq, Frequency Upconversion in Rare-Earth Doped Fluoroindate Glasses. C. R. Chim. 5, 885 (2002).
M. Yamane, and Y. Asahara, Glasses for Photonics (Cambridge: Cambridge University Press, 2000).
H.R. Fernandes, S. Kapoor, Y. Patel, K. Ngai, K. Levin, Y. Germanov, L. Krishtopa, S. Kroeker, and A. Goel, Composition-Structure-Property Relationships in Li2O–Al2O3–B2O3 Glasses. J. Non-Cryst. Solids 502, 142 (2018).
E. Lallier, Rare-Earth-Doped Glass and LiNbO 3 Waveguide Lasers and Optical Amplifiers. Appl. Opt. 31, 5276 (1992).
S. Kaczmarek, Li2B4O7 Glasses Doped with Cr Co, Eu and Dy. Opt. Mater. 19, 189 (2002).
Y. Guan, Z. Wei, Y. Huang, R. Maalej, and H.J. Seo, 1.55 μm Emission and Upconversion Luminescence of Er3+-Doped Strontium Borate Glasses. Ceram. Int. 39, 7023 (2013).
L. Feng, Y. Wu, Z. Liu, and T. Guo, Optical Transitions of Tm3+ in Oxyfluoride Glasses and Compositional and Thermal Effect on Upconversion Luminescence of Tm3+/Yb3+-Codoped Oxyfluoride Glasses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 118, 192 (2014).
M. Abdel-Baki, and F. El-Diasty, Oxyfluoroborate Host Glass for Upconversion Application: Phonon Energy Calculation. Opt. Rev. 23, 284 (2016).
B. Zhou, L. Tao, C.Y.-Y. Chan, Y.H. Tsang, W. Jin, and E.Y.-B. Pun, Intense Near-Infrared Emission of 1.23 μm in Erbium-Doped Low-Phonon-Energy Fluorotellurite Glass. Spectrochim. Acta A Mol. Biomol. Spectrosc. 111, 49 (2013).
F. Qi, F. Huang, L. Zhou, Y. Tian, R. Lei, G. Ren, J. Zhang, L. Zhang, and S. Xu, Long Lifetime of Er3+: 4I11/2 in low Phonon-Energy Fluoro-Chloride Glasses for Mid-Infrared Optical Applications. J. Alloys Compd. 731, 418 (2018).
M. Marzouk, H. ElBatal, and R. Elwan, Effect of MoO3, MnO2 or Mixed Dopants on the Spectral Properties and Crystallization Behavior of Sodium Phosphate Glasses Containing Either MgO or MgF2. Appl. Phys. A 125, 1 (2019).
K.M. Al-Mokhtar, FTIR and Optical Absorption Studies of [B2O3]-[Na2O]-[Li2O]-[CuO] Doped with [MgF2] Nanoparticle. J. Nanotech. Adv. Mat. 8, 45 (2020).
A. El-Daly, M. Abdo, H. Bakr, and M. Sadeq, Impact of Cobalt Ions on the Phonon Energy and Ligand Field Parameters of Some Borate Glasses. J. Non-Cryst. Solids 555, 120535 (2021).
B. Warren, Summary of Work on Atomic Arrangement in Glass. J. Am. Ceram. Soc. 24, 256 (1941).
W. Rittisut, N. Wantana, Y. Ruangtaweep, S. Rujirawat, P. Manyum, R. Yimnirun, P. Kidkhunthod, A. Prasatkhetragarn, S. Kothan, and H. Kim, The Radioluminescence and Photoluminescence Behaviour of Lithium Alumino Borate Glasses Doped with Tb2O3 and Gd2O3 for Green Luminescence Applications. Opt. Mater. 121, 111437 (2021).
E. Kamitsos, Infrared Studies of Borate Glasses. Phys. Chem. Glas. 44, 79 (2003).
F. El Batal, A. El Kheshen, M. Azooz, and S. Abo-Naf, Gamma Ray Interaction with Lithium Diborate Glasses Containing Transition Metals Ions. Opt. Mater. 30, 881 (2008).
M. Azooz, M. Ouis, and H. ElBatal, Preparation and Characterization of Invert Glasses with High CdO Content. J. Non-Cryst. Solids 515, 82 (2019).
B.D. Viezbicke, S. Patel, B.E. Davis, and D.P. Birnie III., Evaluation of the Tauc Method for Optical Absorption Edge Determination: ZnO Thin Films as a Model System. Phys. Status Solidi 252, 1700 (2015).
S. Jat, R.K. Sharma, S. Mahajan, M. Ashiq, and G.F. Ansari, Synthesis and Optical Properties of Ternary TeO2-Bi2O3-Na2O Glass System. Mater. Today: Proc. 42, 1329–1332 (2021).
K. Shinozaki, S. Sukenaga, H. Shibata, and T. Akai, Effect of Mg2+ and Fluorine on the Network and Highly Efficient Photoluminescence of Eu3+ Ion in MgF2–BaO–B2O3 Glasses. J. Am. Ceram. Soc. 102, 2531–2541 (2019).
E. Erdoğan, J. Sci. Technol., Erzincan Üniversitesi Fen Bilimleri Enstitüsü Dergisi, 13, 1 (2020)
M.A. Ragab, F.A. El Yazbi, E.M. Hassan, E.F. Khamis, and M.M. Hamdy, Spectrophotometric Analysis of Two Eye Preparations, Vial and Drops, Containing Ketorolac Tromethamine and Phenylephrine Hydrochloride Binary Mixture and Their Ternary Mixture with Chlorphenirmaine Maleate. Bull. Fac. Pharm. 56, 91 (2018).
T. Som, and B. Karmakar, Structure and Properties of Low-Phonon Antimony Glasses and Nano Glass-Ceramics in K2O–B2O3–Sb2O3 System. J. Non-Cryst. Solids 356, 987 (2010).
S.A. Issa, M. Rashad, H.M. Zakaly, H. Tekin, and A. Abouhaswa, Nb2O5-Li2O-Bi2O3-B2O3 Novel Glassy System: Evaluation of Optical, Mechanical, and Gamma Shielding Parameters. J. Mater. Sci.: Mater. Electron. 31, 22039 (2020).
Acknowledgment
The authors extend their sincere appreciation to the National Research Centre in Egypt. Also, the authors would like to thank Prof. Fouad El-Diasty for his valuable discussion.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-baki, M., Mostafa, A.M., Azooz, M.A. et al. Magnesium Fluoride Borate Glasses for Low Phonon Energy. J. Electron. Mater. 51, 5042–5049 (2022). https://doi.org/10.1007/s11664-022-09742-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09742-0