Abstract
2D Bi2S3/CdS nanosheet arrays have been constructed by a simple three-step method. Firstly, BiOI nanosheet arrays have been electrochemically grown on the surface of conductive FTO substrate and then converted into Bi2S3 nanosheet arrays by ion exchange. Finally, CdS was hydrothermally deposited onto the surface of Bi2S3 nanosheet arrays to form hybrid Bi2S3/CdS nanosheet arrays. The obtained hybrid heterojunction arrays have been used as photoanodes for photoelectrochemical hydrogen evolution and showed enhanced performance and prolonged stability. The photocurrent density of the elegant Bi2S3/CdS nanosheet arrays reaches 9.48 mA/cm2 at 1.23 VRHE under an illumination of 100 mW/cm2 from AM 1.5G sun simulator, which is more than ten times higher than that of the pure Bi2S3 nanosheet arrays and the photocurrent density does not decline obviously after 4 h of continuous operation. Ultimately, a rational mechanism is proposed to elucidate the high performance and excellent stability of Bi2S3/CdS nanosheet arrays for photoelectrochemical cells.
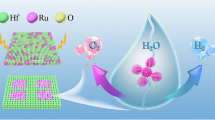
Graphic Abstract
To obtain nanosheet arrays: Arrays of Bi2S3/CdS nanosheets on a FTO substrate are synthesized by electrodeposition, ion exchange and hydrothermal process. The Bi2S3/CdS nanosheet photoanodes show better photoactive and photostability compared to bare Bi2S3 (CdS) nanosheets for photoelectrochemical splitting of water.

Similar content being viewed by others
References
K.N. Ferreira, T.M. Iverson, M. Karim, B. James, and I. So, Science 303, 1831 (2004).
L. Gibson, E.N. Wilman, and W.F. Laurance, Trends Ecol. Evol. 32, 12 (2017).
H. Wang, Y. Yang, and L. Guo, Adv. Energy Mater 7, 1601709 (2016).
S.Z. Wei, J. Kibsgaard, C.F. Dickens, I. Chorkendorff, J.K. Norskov, and T.F. Jaramillo, Science 355, eaad4998 (2017).
M. Zhu, P. Chen, and M. Liu, ACS Nano 5, 4529 (2011).
Y. Yin, N. Ma, J. Xue, G. Wang, S. Liu, H. Li, and P. Guo, Langmuir 35, 787 (2019).
Y. Li, M. Yang, Z. Tian, N. Luo, Y. Li, H. Zhang, A. Zhou, and S. Xiong, Front. Chem. 7, 334 (2019).
H. Wang, P. Hu, J. Yang, G. Gong, L. Guo, and X. Chen, Adv. Mater. 27, 2348 (2015).
C. Pan, X. Jing, Y. Wang, L. Di, and Y. Zhu, Adv. Funct. Mater. 22, 1518 (2012).
Y. Li, J. Feng, H. Li, X. Wei, R. Wang, and A. Zhou, Int. J. Hydrog. Energ. 41, 4096 (2016).
P. Zhang, J. Zhang, and J. Gong, Chem. Soc. Rev. 43, 4395 (2014).
A. Fujishima and K. Honda, Nature 238, 37 (1972).
X. Chen, S. Shen, L. Guo, and S. Samuel, Chem. Rev. 110, 6503 (2010).
M. Marandi, E. Rahmani, and F. Ahangarani, J. Electron. Mater. 46, 6769 (2017).
P. Chen, Z. Liu, X. Geng, J. Wang, M. Zhang, and J. Liu, J. Electron. Mater. 46, 1 (2016).
P.K. Sarswat, N. Deka, S. Rao, L. Free, and G. Kumar, J. Electron. Mater. 46, 1 (2017).
D.J. Desale, S. Shaikh, A. Ghosh, R. Birajadar, F. Siddiqui, A. Ghule, and R.B. Sharma, Compos. Part B 43, 1095 (2012).
S. Misra and H.C. Padhi, J. Appl. Phys. 75, 4576 (1994).
T. Cao, Y. Li, C. Wang, Z. Zhang, and Y. Liu, J. Mater. Chem. 21, 6922 (2011).
K. Lv, Q. Xiang, and J Yu, Appl. Catal. B Environ. 104, 275 (2011).
H. Zhao, Z. Dai, X. Xu, J. Pan, and J. Hu, ACS Appl. Mater. Inter. 10, 23074 (2018).
Y. Shi, Y. Li, X. Wei, J. Feng, and A. Zhou, J. Electron. Mater. 46, 6878 (2017).
H. Li, Y. Gao, Y. Zhou, F. Fan, Q. Han, Q. Xu, X. Wang, M. Xiao, C. Li, and Z. Zou, Nano Lett. 16, 5547 (2016).
Y. Li, R. Wang, H. Li, X. Wei, J. Feng, K. Liu, Y. Dang, and A. Zhou, J. Phys. Chem. C 119, 20283 (2015).
Y. Li, X. Wei, B. Zhu, H. Wang, Y. Tang, and T.C. Sum, Nanoscale 8, 11284 (2016).
J.R. Maiolo, B.M. Kayes, M.A. Filler, M.C. Putnam, M.D. Kelzenberg, H.A. Atwater, and N.S. Lewis, J. Am. Chem. Soc. 129, 12346 (2007).
Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, and H. Yan, Adv. Mater. 15, 353 (2010).
C. Liu, Y. Yang, W. Li, J. Li, Y. Li, and Q. Chen, Int. J. Hydrog. Energ. 41, 5878 (2016).
L. Li, N. Sun, Y. Huang, Q. Yao, N. Zhao, J. Gao, M. Li, H. Zhou, and L. Qi, Adv. Funct. Mater. 18, 1194 (2010).
R. Xing, D. Li, C. An, L. Zhang, Q. Li, and S. Liu, J. Nanosci. Nanotechnol. 12, 8029 (2012).
A.A. Tahir, M.A. Ehsan, M. Mazhar, K.G. Wijayantha, M. Zeller, and A.D. Hunter, Chem. Mater. 22, 5084 (2010).
C. Ludovico, M. Reihaneh, P. Brien, M. Andrea, P. Srebri, N. Kherani, and G. Ozin, Angew. Chem. 39, 20070534 (2010).
X. Wang, J. Xie, and C. Li, J. Mater. Chem. A 3, 1235 (2014).
J. Huang, H. Zhang, X. Zhou, and X. Zhong, Mater. Chem. Phys. 138, 755 (2013).
B. Weng, X. Zhang, N. Zhang, Z.R. Tang, and Y.J. Xu, Langmuir 31, 4314 (2015).
S. Luo, F. Qin, Y. Ming, H. Zhao, Y. Liu, and R. Chen, J. Hazard. Mater. 340, 253 (2017).
L. Hao, G. Chen, Y. Yu, Y. Zhou, Z. Han, and Y. Liu, Int. J. Hydrog. Energ. 39, 14479 (2014).
A. Jana, P. Hazra, M. Hazra, and J. Datta, Mater. Chem. Phys. 183, 173 (2016).
P. Li, X. Zhang, C. Hou, Y. Chen, and T. He, Appl. Catal. B Environ. 238, 656 (2018).
H. He, S.P. Berglund, P. Xiao, W.D. Chemelewski, Y. Zhang, and C.B. Mullins, J. Mater. Chem. A. 1, 12826 (2013).
A. Helal, F.A. Harraz, A.A. Ismail, T.M. Sami, and I.A. Ibrahim, Appl. Catal. B Environ. 213, 18 (2017).
S.U. Shaikh, F.Y. Siddiqui, F. Singh, P.K. Kulriya, D.M. Phase, and R. Sharma, Mater. Chem. Phys. 169, 6 (2016).
W.T. Sun, Y. Yu, H.Y. Pan, X.F. Gao, Q. Chen, and L.M. Peng, J. Am. Chem. Soc. 130, 11240 (2009).
A.B. Wong, S. Brittman, Y. Yu, N.P. Dasgupta, and P. Yang, Nano Lett. 15, 4096 (2015).
Y. Li, X. Wei, H. Li, R. Wang, J. Feng, H. Yun, and A. Zhou, RSC Adv. 5, 14074 (2015).
Y. Yan, Z. Zhou, W. Li, Y. Zhu, Y. Cheng, F. Zhao, and J. Zhou, RSC Adv. 4, 38558 (2014).
I.M. Kobasa and G.P. Tarasenko, Theor. Exp. Chem. 38, 255 (2002).
K.T. Woo and K.S. Choi, Science 343, 990 (2014).
F. Zhen, Y. Liu, Y. Fan, Y. Ni, X. Wei, K. Tang, J. Shen, and C. Yuan, J. Phys. Chem. C 115, 281 (2011).
Acknowledgments
This research is supported by the National Natural Science Foundation of China (No. 51674194, 51074122) and the financial support from Beijing National Laboratory for Molecular Sciences (BNLMS201825) and the ACS Key Laboratory of Colloids, Interfaces and Thermodynamics. We also thank Prof. Kaiqiang Liu and Mr. Xiangyang Yan (School of Chemistry and Chemical Engineering, Shaanxi Normal University) for their technical support in TEM and XPS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Shi, Y., Li, Y. et al. Construction of 2D Bi2S3/CdS Nanosheet Arrays for Enhanced Photoelectrochemical Hydrogen Evolution. J. Electron. Mater. 48, 6397–6405 (2019). https://doi.org/10.1007/s11664-019-07447-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07447-5