Abstract
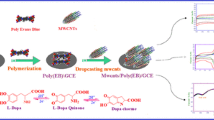
The study demonstrates the potential application of caffeic acid-functionalized magnetite nanoparticles (CA-Fe3O4 NPs) as an effective electrode modifying material for the electrochemical oxidation of the 6-thioguanine (6-TG) drug. The functionalized Fe3O4 NPs were prepared using simple wet-chemical methodology where the used caffeic acid acted simultaneously as growth controlling and functionalizing agent. The study discusses the influence of an effective functionalization on the signal sensitivity observed for the electro-oxidation of 6-TG over CA-Fe3O4 NPs in comparison to a glassy carbon electrode modified with bare and nicotinic acid (NA)-functionalized Fe3O4 NPs. The experiment results provided sufficient evidence to support the importance of favorable functionality to achieve higher signal sensitivity for the electro-oxidation of 6-TG. The presence of favorable interactions between the active functional moieties of caffeic acid and 6-TG synergized with the greater surface area of magnetic NPs produces a stable electro-oxidation signal within the working range of 0.01–0.23 μM with sensitive up to 0.001 μM. Additionally, the sensor showed the strong anti-interference potential against the common co-existing drug molecules such as benzoic acid, acetaminophen, epinephrine, norepinephrine, glucose, ascorbic acid and l-cysteine. In addition, the successful quantification of 6-TG from the commercial tablets obtained from local pharmacy further signified the practical capability of the discussed sensor.
Similar content being viewed by others
References
E. Mirmomtaz, A.A. Ensafi, and H.K. Maleh, Electroanalytical 20, 1973 (2008).
A.P. Subramanian, S.K. Jaganathan, A. Manikandan, K.N. Pandiaraj, N. Gomathi, and E. Supriyanto, RSC Adv. 6, 48294 (2016).
A.P. Subramanian, A.A. John, M.V. Vellayappan, A. Balaji, S.K. Jaganathan, A. Manikandan, and E. Supriyanto, Curr. Sci. 112, 1839 (2017).
A.A. Ensafi and H.K. Maleh, J. Electroanal. Chem. 640, 75 (2010).
H.K. Maleh, A.F. Shojaei, K. Tabatabaeian, F. Karimi, S. Shakeri, and R. Moradi, Biosens. Bioelectron. 86, 879 (2016).
M.M. Ardakani, M.A.S. Mohseni, and M.S. Niasari, Electroanalytical 28, 1370 (2016).
H. Beitollahi, S.G. Ivari, and M. Torkzadeh-Mahani, Mater. Sci. Eng. C 69, 128 (2016).
B. Fang, G. Wang, W. Zhang, M. Li, and X. Kan, Electroanalytical 17, 744 (2005).
P. Posocco, Y.M. Hassan, I. Barandiaran, G. Kortaberria, S. Pricl, and M. Fermeglia, J. Phys. Chem. C 120, 7403 (2016).
K.R. Reddy, K.P. Lee, and A.I. Gopalan, J. Appl. Polym. Sci. 106, 1181 (2007).
K.R. Reddy, B.C. Sin, C.H. Yoo, W. Park, K.S. Ryu, J.S. Lee, D. Sohn, and Y. Lee, Scr. Mater. 58, 1010 (2008).
G. Mathubala, A. Manikandan, S. Arul Antony, and P. Ramar, J. Mol. Struct. 1113, 79 (2016).
S. Güner, Md. Amir, M. Geleri, M. Sertkol, and A. Baykal, Ceram. Int. 41, 10915 (2015).
Md Amir, B. Ünal, M. Geleri, H. GÜngÜneş, Sagar E. Shirsath, and A. Baykal, Superlattices Microst. 88, 450 (2015).
K. Chinnaraj, A. Manikandan, P. Ramu, S. Arul Antony, and P. Neeraja, J. Magn. Magn. Mater. 28, 179 (2015).
A. Manikandan, L. John Kennedy, J. Arul Mary, A. Dinesh, and J. Judith Vijaya, J. Ind. Eng. Chem. 20, 2077 (2014).
A.A. Ensafi, P. Nasr-Esfahani, E. Heydari-Bafrooei, and B. Rezaei, Talanta 131, 149 (2015).
R.A. Dar, P.K. Brahman, S. Tiwari, and K.S. Pitre, Colloids Surf. B 91, 10 (2012).
A. Baykal, H. Erdemi, and M. Amir, J. Inorg. Organomet. Polym. 26, 190 (2016).
U. Kurtan, M. Amir, and A. Baykal, Chin. J. Catal. 36, 705 (2015).
A.V. Anupama, W. Keune, and B. Sahoo, J. Magn. Magn. Mater. 439, 156 (2017).
R.A. Soomro, Z.H. Ibupoto, S.T.H. Sherazi, M.I. Abro, M. Willander, S.A. Mahesar, and N.H. Kalwar, Mater. Express 5, 437 (2015).
Md Amir, M. Geleri, S. Güner, A. Baykal, and H. Sözeri, J. Inorg. Organomet. Polym Mater. 25, 1111 (2015).
Md. Amir, A. Baykal, S. Guner, H. Gungunes, and H. Sozeri, Ceram. Int. 42, 5650 (2016).
V. Jagadeesha Angadi, A.V. Anupama, R. Kumar, S. Matteppanavar, B. Rudraswamy, and B. Sahoo, J. Alloys Comp. 682, 263 (2016).
K.R. Reddy, K.-P. Lee, A.I. Gopalan, and H.-D. Kang, React. Funct. Polym. 67, 943 (2007).
M. Hassan, K.R. Reddy, E. Haque, A.I. Minett, and V.G. Gomes, J. Colloids Interface Sci. 410, 43 (2013).
S.J. Ahn, D.H. Son, and K. Kim, J. Mol. Struct. 324, 223 (1994).
Y.T. Tao, J. Am. Chem. Soc. 115, 4350 (1993).
H. Beitollahi, M.A. Taher, F. Mirrahimi, and R. Hosseinzadeh, Mater. Sci. Eng. C 33, 1078 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amir, M., Tunesi, M.M., Soomro, R.A. et al. Sensitive Determination of 6-Thioguanine Using Caffeic Acid-functionalized Fe3O4 Nanoparticles as an Electrochemical Sensor. J. Electron. Mater. 47, 2198–2208 (2018). https://doi.org/10.1007/s11664-018-6076-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6076-1