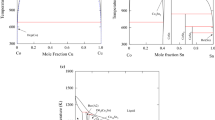
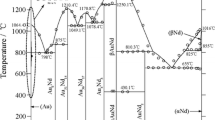
Phase relationships in the Au-Co-Sn ternary system have been thermodynamically assessed by using the CALPHAD technique. The existing thermodynamic descriptions of the binary Au-Sn and Co-Sn systems were improved by incorporating the ab initio calculated enthalpies of formation of the intermetallic compounds including AuSn, CoSn, AuSn2, and AuSn4. For consistency, the Au-Co system was reassessed on the basis of the same pure element data as adopted for the Au-Sn and Co-Sn systems. With the combination of the three binary descriptions, the Au-Co-Sn ternary system was assessed by taking into account the ternary solubility in the binary compounds and the formation of a ternary compound. The obtained set of thermodynamic parameters can reproduce the measured phase equilibria at 380°C. The isothermal section at 396°C, the CoSn-Au and Au-SnCo vertical sections, and the liquidus projection were also calculated.
Similar content being viewed by others
References
R.J. Klein Wassink, Soldering in Electronics, 2nd ed. (Port Erin, Isle of Man: Electrochemical Publications, 1989).
K.N. Tu and K. Zeng, Mater. Sci. Eng. R. 34, 1 (2001).
R.J. Fields, S.R. Low III, and G.K. Lucey, Metal Science of Joining, ed. M.J. Cieslak, J.H. Perepezko, S. Kang, and M.E. Glicksman (Warrendale, PA: TMS, 1992), p. 165.
S.F. Dirnfeld and J.J. Ramon, Weld. J. 69, 373 (1990).
C.Y. Liu, C. Chen, A.K. Mal, and K.N. Tu, J. Appl. Phys. 85, 3882 (1999).
J. Kim, D. Kim, and C.C. Lee, IEEE Trans. Adv. Packag. 29, 473 (2006).
J. Kim and C.C. Lee, Mater. Sci. Eng. A 417, 143 (2006).
J.H. Kuang, M.T. Sheen, C.H. Chang, C.C. Chen, G.L. Wang, and W.H. Cheng, IEEE Trans. Adv. Packag. 24, 563 (2001).
G. Elger, M. Hutter, H. Oppermann, R. Aschenbrenner, H. Reichl, and E. Jäger, Microsyst. Technol. 7, 239 (2002).
J.W.R. Tew, X.Q. Shi, and S. Yuan, Mater. Lett. 58, 2695 (2004).
T. Yamamoto, S. Sakatani, S. Kobayashi, K.F. Keisuke, M. Ishio, and K. Shiomi, Mater. Trans. JIM 46, 2406 (2005).
W.J. Zhu, H.S. Liu, J. Wang, and Z.P. Jin, J. Alloys Compd. 456, 113 (2008).
F. Gao, T. Takemoto, and H. Nishikawa, Mater. Sci. Eng. A 420, 39 (2006).
L. Liu, C. Anderson, and J. Liu, J. Electron. Mater. 33, 935 (2004).
C.P. Vassilev, K.I. Lilova, and J.C. Gachon, Intermetallics 15, 1156 (2007).
T. Laurila, V. Vuorinen, and J.K. Kivilahti, Mater. Sci. Eng. R 49, 1 (2005).
C.W. Huang and K.L. Lin, J. Electron. Mater. 35, 2135 (2006).
A. Neumann, A. Kjekshus, C. Rømming, and E. Røst, J.␣Alloys Compd. 240, 42 (1996).
K.C. Hari Kumar, P. Wollants, and L. Dalaey, CALPHAD 18, 71 (1994).
G. Kresse and J. Furthmuller, Phys. Rev. B 54, 11169 (1996).
G. Kresse and J. Furthmuller, Comput. Mater. Sci. 6, 15 (1996).
M. Jiang, J. Sato, I. Ohnuma, R. Kainuma, and K. Ishida, CALPHAD 28, 213 (2004).
H.S. Liu, C.L. Liu, K. Ishida, and Z.P. Jin, J. Electron. Mater. 3, 1290 (2003).
J.O. Anderson, T. Helander, L. Hoglund, P. Shi, and B. Sundman, CALPHAD 26, 273 (2002).
PANDAT software for multicomponent phase diagram calculations by CompuTherm. (LLC, Madison, WI, since 2000).
V. Grolier and R. Schmid-Fetzer, Int. J. Mater. Res. 98, 797 (2007).
H. Okamoto, T.B. Massalski, M. Hasebe, and T. Nishizawa, Bull. Alloy Phase Diagrams 6, 449 (1985).
J. Korb, unpublished assessment, GTT-Technologies, (2004).
A.T. Dinsdale, CALPHAD 15, 317 (1991).
W. Wahl, Z. Anorg. Chem. 66, 60 (1910).
U. Hashimoto, J. Jpn. Inst. Met. 1, 177 (1937).
E. Raub and P. Walter, Z. Metallkd. 41, 234 (1950).
A.T. Grigor’ev, E.M. Sokolovskaya, and M.V. Maksimova, Zh. Neorg. Khim. 1, 1047 (1956).
L. Weil, Z. Phys. Chem. 16, 368 (1958).
P. Taskinen, Scand. J. Met. 13, 39 (1984).
W. Klement Jr., Trans. Met. Soc. AIME 227, 965 (1963).
V.V. Berezutskiy, V.N. Eremenko, and G.M. Lukashenko, Izvest. Akad.Nank SSSR Metally 54 (1975).
A. Kubil and C.B. Alcol, Met. Sci. J. 1, 19 (1967).
S.S. Wang and J.M. Toguri, Can. J. Chem. 51, 2362 (1973).
B. Predel and E. Zehnpfund, Z. Metallkd. 64, 782 (1973).
L. Topor and O.J. Kleppa, Metall. Trans. B 15, 573 (1984).
P.E. Blöchl, Phys. Rev. B 50, 17953 (1994).
G. Kresse and J. Joubert, Phys. Rev. B 59, 1758 (1999).
J.P. Perdew and Y. Wang, Phys. Rev. B 45, 13244 (1992).
J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, and D.J. Singh. Phys. Rev. B 46, 6671 (1992).
H.J. Monkhorst and J.D. Pack, Phys. Rev. B 13, 5188 (1976).
M. Methfessel and A.T. Paxton, Phys. Rev. B 40, 3616 (1989).
S. Lidin and A.K. Larsson, J. Solid State Chem. 118, 313 (1995).
A.K. Jena and M.B. Bever, Metall. Trans. B 10, 545 (1979).
H. Okamoto, J. Phase Equilib. 14, 765 (1993).
K. Zeng and J.K. Kivilahti, J. Electron. Mater. 30, 35 (2001).
C.L. Liu, Z.P. Jin, and H.S. Liu, Chin. J. Nonferr. Met. 13, 1343 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, H.Q., Jin, S., Zhang, L.G. et al. Thermodynamic Assessment of the Au-Co-Sn Ternary System. J. Electron. Mater. 38, 2158–2169 (2009). https://doi.org/10.1007/s11664-009-0874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-009-0874-4