Abstract
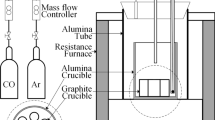
This study aimed to investigate the thermodynamic characteristics of the reaction between Al and B2O3 in the continuous casting process of high-aluminum steel. The activity coefficient of B2O3 in molten slag of (31.1 to 58.4) pct CaO–(0.2 to 16.7) pct SiO2–(14.7 to 49.2) pct Al2O3–(0 to 30.3) pct B2O3–(0 to 10.5) pct CaF2–(2.1 to 4.7) pct MgO–(0 to 1.8) pct MnO slags was measured at 1450°C using B equilibrium experiments between liquid copper and molten slag in a graphite crucible under the mixed gas atmosphere of CO and Ar. The effects of B2O3, Al2O3, SiO2, and CaF2 and the basicity (B = mass pct CaO/(mass pct SiO2+mass pct Al2O3) on the activity coefficient of B2O3 in molten slag were discussed. Regression analysis was used to investigate the quadratic relationship between the activity coefficient of B2O3 and the concentrations of components in the slag. The results indicated that (a) the activity coefficient of B2O3 increased with an increase in B2O3 when B2O3 > 7 pct and B < 1, but decreased when B2O3 > 7 pct and B > 1. (b) When B > 0.9 and Al2O3 > 26 pct, the activity coefficient of B2O3 went up with the increase of Al2O3, but decreased when B < 0.9. (c) When B = 0.93 to 0.97, B2O3 = 9.1 to 9.5 pct, and CaF2 < 11 pct, the activity coefficient of B2O3 decreased with an increase in CaF2. (d) When B = 0.8 to 1.3, the activity coefficient of B2O3 increased with the increase of the basicity B. These findings provide insight into the factors affecting the activity coefficient of B2O3 in the molten slag and contribute to the optimization of the continuous casting process of high-aluminum steel.









Similar content being viewed by others
References
L. Zhou and W. Wang: Metall. Mater. Trans. E, 2016, vol. 3E, pp. 139–44.
J.-Y. Park, G.H. Kim, J.B. Kim, S. Park, and I. Sohn: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 2582–94.
L. Zhang, W.-L. Wang, and H.-Q. Shao: J. Iron Steel Res. Int., 2019, vol. 26, pp. 336–44.
Z. Wang and I. Sohn: ISIJ Int., 2020, vol. 60, pp. 2705–16.
M.-S. Kim, M.-S. Park, and Y.-B. Kang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2077–82.
M. Ueno and T. Inoue: Trans. ISIJ, 1973, vol. 13, pp. 211–17.
L. Qi, A.-M. Zhao, and Z.-Z. Zhao: Adv. Mater. Res., 2011, vol. 233–235, pp. 1063–66.
J. Takahashi, K. Ishikawa, K. Kawakami, M. Fujioka, and N. Kubota: Acta Mater., 2017, vol. 133, pp. 41–54.
N. Dudova, R. Mishnev, and R. Kaibyshev: ISIJ Int., 2011, vol. 51(11), pp. 1912–18.
A.A. Azarkevich, L.V. Kovalenko, and V.M. Krasnopoiskii: Met. Sci. Heat Treat., 1995, vol. 37, pp. 22–24.
T. Kasuya and Y. Hashiba: Sci. Technol. Weld. Join., 1999, vol. 4(5), pp. 265–75.
T. Kamo, M. Hamada, and Y. Komizo: Bull. Chem. Soc. Jpn., 2002, vol. 20(2), pp. 276–81.
Z.-C. Wang, S.-X. Tong, X. Liu, Y. Su, and F. Cao: J. Chem. Thermodyn., 1995, vol. 27, pp. 873–78.
Z.-C. Wang, Y. Su, and S.-X. Tong: J. Chem. Thermodyn., 1996, vol. 28, pp. 1109–13.
Z.-Q. Huang, Z.-P. Yang, Y. Su, S.-X. Tong, and Z.-C. Wang: J. Chem. Thermodyn., 1995, vol. 27, pp. 1429–32.
A.S. Sunkar and K. Morita: ISIJ Int., 2009, vol. 49, pp. 1649–55.
X. Huang, T. Fujisawa, and C. Yamauch: ISIJ Int., 1996, vol. 36, pp. 133–37.
X.-M. Huang, K. Asano, T. Fujisawa, Z. Sui, and C. Yamauch: ISIJ Int., 1996, vol. 36, pp. 1360–65.
M. Sakamoto, Y. Yanaba, H. Yamamura, and K. Morita: ISIJ Int., 2013, vol. 53, pp. 1143–51.
L.A.V. Teixeira, Y. Tokuda, T. Yoko. and K. Morita: ISIJ Int., 2009, vol. 49, pp. 777–82.
I.Y. Archakov, V.L. Stolyarova, and M.M. Shultz: Rapid Commun. Mass Spectrom., 1998, vol. 12, pp. 1330–34.
I.A. Sobolev, F.A. Lifanov, S.V. Stefanovskii, S.A. Dmitriev, V.N. Zakharenko, and A.P. Kobelev: Glass Ceram., 1987, vol. 44, pp. 51–54.
H. van Limpt, R. Beerkens, and S. Cook: Eur. J. Glass Sci. Technol., 2011, vol. 52(3), pp. 77–87.
J.-B. Chen, W.-B. Pan, H.-H. Huang, Z.-Y. Chen, M.-H. Zhao, and Y.-Q. Sun: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 1526–37.
J.-B. Chen, H.-Z. Luan, H.-H. Huang, M.-H. Zhao, W.-B. Pan, and Z.-Y. Chen: ISIJ Int., 2022, vol. 62, pp. 1341–51.
J.-B. Chen, H.-H. Huang, R. Chu, and Y.-Q. Sun: ISIJ Int., 2021, vol. 61, pp. 1842–49.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, 1980.
K.T. Jacob, S. Priya, and Y. Waseda: Metall. Mater. Trans. A, 2000, vol. 31A, pp. 2674–78.
J. Yang, J.-Q. Zhang, O. Ostrovski, C. Zhang, and D.-X. Cai: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 291–303.
K. Liu, Y.-H. Han, Z.-F. Yuan, L.-G. Zhu, and X.-T. Yu: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 1504–15.
J. Yang, Q. Wang, J.-Q. Zhang, O. Ostrovski, C. Zhang, and D.-X. Cai: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2794–803.
G.-H. Kim and I. Sohn: J. Am. Ceram. Soc., 2019, vol. 102, pp. 6575–90.
M. Kowalski, P.J. Spencer, and D. Neuschütz: Slag Atlas, 2nd ed., Verlag Stahleisen GmbH, Düsseldorf, 1995.
G.K. Sigworth and J.F. Elliott: Met. Sci., 1974, vol. 8, pp. 298–310.
Acknowledgments
The authors express their gratitude to the funding support by National Natural Science Foundation of China (No. 51874198).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Jb., Che, Hj., Zhao, Mh. et al. Thermodynamic Activity of B2O3 in CaO–SiO2–Al2O3–B2O3–MnO–MgO Molten Slags at 1723 K. Metall Mater Trans B 54, 2737–2746 (2023). https://doi.org/10.1007/s11663-023-02870-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02870-w