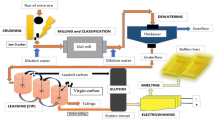
Abstract
The rise of residues derived from hydrometallurgical processes of zinc production has made this industry face numerous challenges. Residues contain elements whose reclamation is of high significance due to the economic improvement of the whole process and the removal of environmental consequences. The previous study examined the selective extraction of Zn and As from hot purification filter cakes of the zinc process and found that the dominant species in arsenic-bearing solution was AsO43−. With a Zn2(AsO4) (OH) composition containing As 23.1 wt pct, As was precipitated with an 95 pct efficiency by adding zinc sulfate under the following conditions: Molar ratio Zn/As = 1, pH 6.5, time = 4 hours, temperature = 80 °C, and stirring velocity = 500 rpm. The zinc arsenate hydroxide was successfully used as an activator of the zinc powder in the cobalt cementation process. The examination of the precipitate revealed that As increased the anodic and cathodic reactions potential difference. Thus, thermodynamic driving force of the reaction increased and cementation improved by forming intermediate compounds, like CoAs, with higher stability and more positive reduction potential.













Similar content being viewed by others
References
S. Choi, K. Yoo, R.D. Alorro, and C.B. Tabelin: Miner. Eng., 2020, vol. 145, p. 106061.
J. Lu, D. Dreisinger, and K. Rees: Hydrometallurgy, 2020, vol. 197, p. 105479.
B. Behnajady, A.A. Balesini, and J. Moghaddam: Can. Metall. Q., 2014, vol. 53(3), pp. 333–39.
V. Vahidfard, K. Shayesteh, P. Abbasi, and M. Hosseini: J. Part. Sci. Technol., 2021, vol. 6(2), pp. 81–93.
P. Abbasi, K. Shayesteh, V. Vahidfard, and M. Hosseini: Iran. J. Anal. Chem., 2021, vol. 8(1), pp. 17–28.
P. Abbasi, K. Shayesteh, V. Vahidfard, and M. Hosseini: Iran. J. Chem. Eng., 2020, vol. 17(4), pp. 3–20.
K. Shayesteh, P. Abbasi, V. Vahidfard, and M. Shahediasl: Arabian J. Sci. Eng., 2020, vol. 45(2), pp. 587–98.
D.C.A. Gonçalves, D. Majuste, and V.S.T. Ciminelli: Hydrometallurgy, 2021, vol. 201, p. 105572.
B. Krause and R. Sandenbergh: Hydrometallurgy, 2015, vol. 155, pp. 132–40.
B. Behnajady and J. Moghaddam: J. Cent. S. Univ., 2015, vol. 22(6), pp. 2066–72.
J. Näsi: Hydrometallurgy, 2004, vol. 73(1), pp. 123–32.
T. Gutknecht, Y. Cao, Y. Colombus, and B.M. Steenari: Hydrometallurgy, 2018, vol. 181, pp. 169–79.
T. Wang, G. Lin, L. Gu, T. Hu, T. Xie, H. Qu, S. Wang, L. Zhang, S. Cheng, J. Liu, and H. Di: Mater. Res. Express, 2019, vol. 6(10), p. 106588.
M.D. Rao, A. Meshram, H.R. Verma, K.K. Singh, and T.R. Mankhand: Hydrometallurgy, 2020, vol. 195, p. 105352.
B. Behnajady and J. Moghaddam: Hydrometallurgy, 2017, vol. 173, pp. 232–40.
B. Behnajady and J. Moghaddam: Chem. Eng. Res. Des., 2017, vol. 117, pp. 564–74.
G. Roman-Ross, G.J. Cuello, X. Turrillas, A. Fernandez-Martinez, and L. Charlet: Chem. Geol., 2006, vol. 233(3–4), pp. 328–36.
K. Tozawa, T. Nishimura, M. Akahori, and M.A. Malaga: Hydrometallurgy, 1992, vol. 30(1–3), pp. 445–61.
K. Tanabe, T. Ohgai, T. Akiyama and H. Fukushima: in Lead and Zinc ’95, 1995, pp. 303–09.
A. Nelson: Novel activators in cobalt removal from zinc electrolyte by cementation, Ph.D. Thesis, Dept. of Mining and Metallurgical Engineering, McGill Univeristy, Montreal, 1998.
P. G. West-Sells: Fundamental study of the deposition of cobalt from electrolytes containing zinc, Ph.D. Thesis, Dept. of Metals and Materials Engineering, University of British Columbia, Vancouver, 1996.
K. Higashi, H. Fukushima, T. Urakawa, T. Adaniya, and K. Matsudo: J. Electrochem. Soc., 1981, vol. 128(10), pp. 2081–85.
O. Bøckman and T. Østvold: Hydrometallurgy, 2000, vol. 54(2), pp. 65–78.
O. Bøckman and T. Østvold: Hydrometallurgy, 2000, vol. 55(1), pp. 107–12.
O. Bøckman, T. Østvold, G.A. Voyiatzis, and G.N. Papatheodorou: Hydrometallurgy, 2000, vol. 55(1), pp. 93–105.
S. Zaheri: High temperature and high pressure cobalt cementation onto zinc dust, M.A.Sc. Thesis, Dept. of Materials Engineering, University of British Columbia, Vancouver, 2015.
R. W. Lew: The removal of cobalt from zinc sulphate electrolyte using the copper-antimony process, M.A.Sc. Thesis, Dept. of Metals and Materials Engineering, University of British Columbia, Vancouver, 1994.
V. van der Pas and D.B. Dreisinger: Hydrometallurgy, 1996, vol. 43(1–3), pp. 187–205.
A. Nelson, W. Wang, G.P. Demopoulos, and G. Houlachi: Miner. Process. Extr. Metall. Rev., 2000, vol. 20(1), pp. 325–56.
K. BØrve and T. Østvold: in Hydrometallurgy’94, Springer, 1994, pp. 563–77.
R. Raghavan, P.K. Mohanan, and S.K. Verma: Hydrometallurgy, 1999, vol. 51(2), pp. 187–206.
D. Yang, G. Xie, G. Zeng, J. Wang, and R. Li: Hydrometallurgy, 2006, vol. 81(1), pp. 62–66.
B.S. Boyanov, V.V. Konareva, and N.K. Kolev: Hydrometallurgy, 2004, vol. 73(1–2), pp. 163–68.
D. Jun, W. De-quan, J. Lan, and J. Man: Trans Nonferrous Met. Soc. China, 2002, vol. 12(6), pp. 1172–75.
T.M. Dreher, A. Nelson, G.P. Demopoulos, and D. Filippou: Hydrometallurgy, 2001, vol. 60(2), pp. 105–16.
A. Dib and L. Makhloufi: Miner. Eng., 2007, vol. 20(2), pp. 146–51.
A. Dib and L. Makhloufi: Chem. Eng. J., 2007, vol. 130(1), pp. 39–44.
A. Dib and L. Makhloufi: Chem. Eng. J., 2006, vol. 123(1–2), pp. 53–58.
J. Lu, Cobalt precipitation by reduction with sodium borohydride, M.A.Sc. Thesis, Dept. of Materials Engineering, University of British Columbia, Vancouver, 1995.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behnajady, B., Moghaddam, J. Synthesizing Zinc Arsenate and Its Application in Cobalt Purification Process. Metall Mater Trans B 54, 1113–1121 (2023). https://doi.org/10.1007/s11663-023-02744-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02744-1