Abstract
The dome zone is the freeboard at the top of the COREX melter gasifier (MG), and the secondary injection systems are installed in the dome zone to recycle dust and oxygen. Pulverized coal injection (PCI) in the dome zone of the COREX MG is used to transform the oxygen burners (OBs) into oxygen–coal burners (OCBs) and cooperates with the dust burners (DBs) to realize the function of gasification. In this work, a three-dimensional (3-D) steady-state mathematical model was developed to simulate the transport phenomenon in the dome zone with and without PCI. The results show that when 150 kg/t of pulverized coal is injected into the dome zone, the impinging stream area expands and the average temperature in the dome zone increases by about 40 K. The proportion of CO2 in the reduction gas decreases by 7.8 pct, while the proportion of H2 increases by 7.4 pct at the outlet of the MG with the PCI in the dome zone. The total effective volume fraction of reducing gas at the outlet of MG can reach 83.3 pct, which is a benefit to improving the reduction processes in the shaft furnace. Due to the intensification of circulation and the complexity of particle behavior, the residence time of particles increases by about 10 seconds in the case of PCI and the burnout rate of recycling dust and pulverized coal can reach above 99 pct.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, great attention is given to global warming and CO2 emission from energy-intensive industries such as power plants and the steel industry.[1,2,3,4] In the latter, the blast furnace is the dominant ironmaking process unit used around the world.[5] A large amount of coke is used in the blast furnace to provide energy and reactants. The coke oven is a major source of CO2 and pollutants. On the other hand, high-quality coking-coal resources are being depleted, resulting in rising coke prices. As a result, in recent decades, smelting reduction ironmaking technology has been developed, which is designed to use less or even zero coke.[6,7] The COREX is a cost-efficient and environmentally friendly process that has been successfully applied in industrial-scale production in South Africa, South Korea, India, and China.[8]
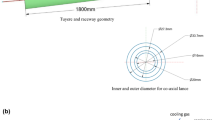
The COREX is a two-stage process that involves pre-reduction in a shaft furnace, followed by final reduction and separation in a melter gasifier (MG), as shown in Figure 1(a). The MG plays a role in providing heat and reducing gas, and it can be divided into the freeboard in the dome zone, packed bed, raceway, and hearth, from top to bottom,[9] as shown in Figure 1(b). Actually, secondary injection systems are installed in the dome zone to recycle dust and oxygen. The oxygen burner in the dome zone can be converted to an oxygen–coal burner (OCB) for pulverized coal injection (PCI) by referring to coal gasification technology. The pulverized coal and oxygen can be injected into the dome zone, allowing gasification to occur when a partial oxidant is supplied. On the premise of meeting the smelting heat demand of the packed bed and hearth zone, the PCI in the dome zone can partially undertake the function of reducing gas generation from lump coal in the lower part of the MG, greatly improving the quality of reducing gas and promoting the reduction process in the shaft furnace. On the other hand, injecting low-cost powdered coal can lower the production cost and control the total fuel ratio in COREX. Hence, it is necessary to study the PCI in the dome zone of the MG for the optimization and development of the COREX process.
The COREX MG is a “black box” reactor, and it is difficult to directly observe and measure the inner characteristics under high-temperature and high-pressure conditions. The numerical simulation method that couples heat, mass, and momentum balance is regarded as an alternative for investigating and optimizing the PCI in the dome zone of the COREX MG. Computational fluid dynamics (CFD) has been widely used to study coal gasification in gasifiers,[10,11,12,13] coal combustion in the raceway of blast furnaces,[14,15,16,17] and typical transport phenomena in metallurgical reactors.[18,19,20] Many CFD codes have been applied to model the COREX MG. For example, Lee et al. developed a one-dimensional (1-D) model of the MG, taking into account the pyrolysis behavior of coal, and the effects of bed height, C/O ratio, top pressure, and steam injection on process performance were studied.[21,22] Pal and Lahiri developed a pseudo–two-dimensional (2-D) model for the COREX MG. The model was developed for the moving bed, where the tuyere region was 2-D and the rest was 1-D.[23] Pal and Lahiri also developed a dynamic model of the COREX MG to study the transient behavior of the furnace. The effects of pulse and step disturbances were investigated.[24] The gas distribution,[25] raceway phenomena,[26] and gas–solid flow[27] in the COREX MG were investigated through a CFD or computational fluid dynamics and discrete element method (CFD-DEM) model. These works played a significant role in helping us understand and improve the COREX MG. However, there is little information about the transport phenomenon in the dome zone of the MG.
The dome zone is a unique characteristic of the COREX process, and the fluctuation of dome temperature is undesirable for maintaining a stable operation. Inadequate dome temperature could lead to the insufficient cracking of the hydrocarbons in the coal and tar concentrations clogging the generator gas nozzle, while excessive freeboard temperature may result in the burnout of the refractory and the device in the freeboard, especially the distribution system. More attention was drawn to the transport phenomenon, including the combustion behavior of carbonic particles in the dome zone of the COREX MG. For example, Berger et al. numerically studied the combustion behavior of recycling carbon dust in front of the dust burner (DB).[28] Du et al. developed a three-dimensional (3-D) mathematical model for the inner characteristics of the freeboard in the dome zone; the recycling dust combustion and the temperature distribution, in particular, were analyzed.[9] Wang et al. used a 3-D mathematical model to focus on the conversion of recycling dust and the effects of carbon dust burning on the dome gas composition.[29] These works, particularly the CFD model, serve as an effective reference for the exploration of PCI in the dome of the COREX MG. However, the different properties between recycling dust and pulverized coal, and the corresponding operation, make it impossible for the latter to draw experience directly from recycling dust injection. Recently, one COREX-3000 module in Baosteel was moved from Shanghai to Xinjiang in a bid to use local coal resources. The dome in the MG has been equipped with four OCBs, four DBs, and two oxygen/top gas burners.[30] The practice of PCI in the dome zone of the COREX MG showed that the amount of generated reducing gas increased by 15 pct and the metallization ratio in the shaft furnace increased by 15 to 20 pct.[31] Xu et al. proposed a smelting reduction ironmaking process coupled with pulverized coal gasification on the upper part of a smelting furnace, and the characteristics of velocity, temperature, gas composition distribution, and gasification performance were numerically studied.[32] However, detailed information about the PCI in the dome zone of the COREX MG is still rare, particularly the quantitative comparisons of multiphase reaction flow and the reduction of gas composition with and without PCI.
In this work, a 3-D mathematical model is developed to study the PCI in the dome zone of the COREX MG. The transport phenomena in the dome zone with and without PCI are compared, with a particular focus on the velocity, temperature, gas composition, and particle behavior. The findings of this work lay a foundation for further research of COREX MG operation of the PCI in the dome zone and provide a theoretical basis for guiding the actual industrial production and application.
Mathematical Model
Governing Equations
In the simulation of the PCI in the dome zone of the COREX MG, the gaseous phase is treated as a continuous phase, with the assumption of an incompressible ideal gas. Three-dimensional, steady-state Reynolds time-averaged Navier–Stokes equations are solved. The governing equations solved for the gas phase include mass, heat, momentum, turbulence kinetic energy, turbulence dissipation rate, and a number of species (O2, N2, CO2, CO, H2, H2O, CH4, and volatiles, as used by others[13,15]). The governing equations can be expressed by the following general partial differential formula.[16,33]
From the left, each term represents the unsteady term, convection term, diffusion term, and source term in turn, where the source term is the term that cannot be included in the first three terms.
The gases are sprayed into the dome zone at a high velocity, and turbulence is fully developed. To predict the turbulent mixing and diffusion trajectory of the particle phase in high-speed flow more accurately and effectively, the realizable k−ε model is used to close the steady-state Reynolds time-averaged Navier–Stokes equations.[10] The transport equations of turbulent kinetic energy k and turbulent dissipation rate ε are shown as follows.
where Gk and Gb are the turbulent kinetic energy term generated by the laminar velocity gradient and buoyancy, respectively. C1 = 1.44, C2 = 1.92, σk = 1, σε = 1.2, σk, and σε are the turbulence Prandtl numbers of the k and ε equations.
The recycling dust and pulverized coal are regarded as discrete particle phases. Due to the volume loading of the particle phase in the dome zone being less than 10 pct, the Lagrangian method is adapted. Once particles enter the dome zone through the burner, they will be rapidly diluted by other gas phase components. Therefore, the interaction between the particles is not considered. The random walk model (RWM) is used to describe the trajectory of particles, and the motion of particles follows Newton’s second law.[34]
where mp is the mass of particle; FD(u–up) is the drag force of particle in per unit mass; up is particle velocity; u is gas velocity; ρp and ρ are the density of particle and gas, respectively; and Fx is the sum of other forces on the particle. FD can be calculated by Eq. [5] where CD is the coefficient of drag force.
There are three main types of heat transfer used to decide the temperature change of particles, including convective heat transfer, sensible heat caused by mass transfer, and radiation heat transfer. The radiation heat transfer is solved by the P-1 radiation model, in which the radiation coefficient is evaluated by the gray-gas-weighted average model.[35]
Chemical Reaction Model
The particle combustion is considered as a four-stage process: (1) preheating, (2) devolatilization, (3) gaseous combustion of volatiles, and (4) oxidation and gasification of residual char in the gas phase.
Devolatilization model
The evaporation process of water is not considered because both dust and pulverized coal have been preheated to above 373 K before entering the dome. It is worth noting that the combustible carbon in the dust particle originates from the unburned coal and coke.[36] Therefore, in addition to pulverized coal, dust particles need to consider the release of volatile in the heating process. The different types of devolatilization models have been proposed using a range of reaction schemes and rate expressions, whereas the two-competing-reactions model proposed by Ubhayakar[37] is widely used to simulate the devolatilization process. It assumes that there is a pair of parallel first-order irreversible reactions in the process of devolatilization, which has been applied in many related simulations.[13,15,16,38]
where reaction rate constants k1 and k2 are expressed by the Arrhenius formula:
Among them, A is the pre-exponential factor and E is the activation energy. For path 1, A and E are 3.7 × 105 s–1 and 1.5 × 108 J/kmol, respectively. For path 2, A and E are 1.46 × 1013 s–1 and 2.51 × 108 J/kmol, respectively. Volatile is an organic compound consisting of various elements, and it can be expressed as CαHβOγNθ. When the temperature condition is satisfied, the decomposition process of volatile is assumed to occur instantaneously. The mixtures of CO, CO2, CH4, H2, and N2 are regarded as the decomposition products of volatile. Based on the elemental analysis of dust and coal, the stoichiometric coefficient of each component can be estimated, and this process can be described as follows.[39]
Homogeneous reactions
The homogeneous reactions involved in the dome can be divided into two categories. One is the oxidation reaction, including the combustion of CO, H2, and CH4, and the other is the reversible gasification reaction between CO, CH4, and steam. Considering the dual effects of turbulent diffusion and the chemical reaction rate, the finite-rate/eddy-dissipation model is used to describe the turbulence-chemistry interaction.[28] The net reaction rate depends on the smaller one of them. Table I shows the homogeneous reactions included in the model and the kinetic parameters of each reaction. The related chemical reactions are as follows.
Heterogeneous reactions
After the release of the volatile, the residual char will participate in the heterogeneous reactions, including the reactions of C(s)-O2, C(s)-CO2, and C(s)-H2O. The multiple surfaces reactions model assumes that the particle surface species can be depleted or produced by the stoichiometry of the particle surface reaction. The particle surface species of dust and pulverized coal are regarded as carbons, participating in oxidation and the gasification reactions of residual char. The Arrhenius formula is used to calculate the kinetic rate of reaction r:
The apparent order of reaction r is 1, so the rate of particle surface carbon depletion Rj,r is given by
where Ap and ηr are the particle surface area (m2) and effectiveness factor, respectively. Yj is the mass fraction of surface j in the particle. Pn represents the bulk partial pressure of the gas phase species n (Pa). The diffusion rate coefficient D0,r, for the reaction r, is defined as Eq. [12]:
where C1,r indicates the molar concentration of species j in reaction r. The related kinetic parameters are summarized in Table I.[10,16]
Except for the reactions mentioned previously, the oxidation reactions of alkalis and reduction reactions of iron oxides are not considered in this simulation. This is because the enthalpies involved in these reactions have little effect on the overall temperature of the dome compared with the combustion or gasification reactions.
Simulation conditions
In this study, the geometric model is constructed regarding COREX-3000 industrial data, as shown in Figure 2. The geometric model is roughly a hemispherical space, with four generator gas outlets in the upper part, which are set as pressure outlets. The upper boundary of the packed bed zone is set as the bottom inlet of the mathematical model, where the rising reducing gas enters the dome zone. There is a row of circumferential burners arranged in the shrink part of the dome, and the angle between the tip of the lance and the horizontal line is 8 deg. For the case of the recycling dust injection in the dome zone (regarded as case 1 in this work), there are six oxygen burners (OBs) and four DBs at the lower part of the geometry. Their distributions and positions in the present study are consistent with those in the literature.[9] Industrial pure oxygen (99.5 pct O2) is introduced by cylindrical hollow pipes in the OB. Meanwhile, the DB is equipped with a coaxial lance, of which the oxygen is transported in an external tube and the dust is injected into the inner tube with a carrier gas (100 pct N2). This structure could promote the formation of a shear layer of oxygen flow, which is conducive to improving the burnout rate of dust particles. For the coal injection in the dome zone (regarded as case 2), four OBs (located at the 22.5 deg, 157.5 deg, 202.5 deg, and 337.5 deg positions along the circumferential direction) are converted to OCBs, and the DB maintains the original structure and position, as shown in Figure 3. The OCB is also a coaxial lance, but, unlike the DB, the oxygen is blown in through the inner tube and the coal particles are injected from the external tube. This structure can prevent excessive oxygen combusting with the rising reducing gas and enable more pulverized coal particles to gasify under insufficient oxidant conditions. All entrances in the model are treated as velocity inlets. The furnace wall is simplified as the nonslip shear condition, and there is no existing mass penetration. In practical production, the burden from the upper shaft furnace charging into the dome zone will take away the heat and the charging lump coal in the top of the dome zone also will consume the heat. In this work, these heats are considered as an energy source term in the governing equation to obtain accurate results. Based on the mass and heat balances model developed in our previous work,[8] the value of the energy source is – 8400 w/m3.
The operation parameters of MG under the condition of recycling dust injection (case 1) and PCI (case 2) are shown in Table II. The oxygen supply in both the DB and OB decreases in case 2, and more oxygen is supplied through the OCB, which could form the temperature and pressure conditions for satisfying the gasification of coal. In addition, the composition of rising gas coming from the packed bed also changes. All of these operating parameters are obtained from the plant operation.
The properties of recycling dust and coal particles are shown in Table III. The proximate and ultimate analyses show that recycling dust is a valuable secondary material.[39] The carbon in the cycling dust mainly originates from unconsumed fine coke from the packed bed and the fine coal from the lump coal. So, the volatiles of recycling dust, approximately 4.6 pct, must be considered in this work. The particle size distribution of the recycling dust is heterogeneous with a range between 5.03 and 91.09 μm. The mean particle size of the recycling dust is 28.88 μm, which is larger than the sintering dust and the electric arc furnace dust, and similar to the blast furnace dust. The properties of coal refer to industrial data. The particle size distribution of pulverized coal changes from 10 to 200 μm, and its mean particle size is about 80 μm. Therefore, in this work, the sizes of recycling dust and coal particles are assumed to be 28.8 and 80 μm, respectively, as shown in Table III. Work on the influence of particle size distribution on the transport phenomena in the dome zone is in progress and will be reported in the near future.
Although this model is developed based on industrial data, some inevitable simplifications are made to improve the efficiency, including the following.
-
(1)
The composition and temperature of the rising gas are regarded as a uniform diffusion when the gas enters into the dome zone.
-
(2)
No adherence or agglomeration occurs during the motion of particles.
-
(3)
Dust particles–wall collisions do not influence particle heat and mass transfer.
-
(4)
CH4 is produced by the decomposition of volatile in the pulverized coal.
The simulation work is completed based on Fluent 19.5. The governing equations, turbulent kinetic energy, turbulent dissipation, and other equations are solved by the finite volume method. The SIMPLE semi-implicit algorithm is used to calculate the pressure–velocity coupled equations. The convergence standard requires the residual curves of each index to be less than 1 × 10–5; the residual curves of energy and P1, especially, are less than 1 × 10–6. At the same time, the variation trend of H2 concentration at the outlet and the dome average temperature to judge the convergence of simulation results are monitored. When the average relative error of variables before and after iteration is less than 0.1 pct, the simulation result is regarded as convergence.
Results and discussion
Model Validation
A mesh-independence test and model validation are conducted before the simulation. Four different mesh resolutions with cell numbers of 214866, 269410, 312024, and 363228 are adopted for the mesh independence analysis. Figure 4 depicts the distribution of the volume fraction of H2 along with the height of the central axis in the dome with different mesh resolutions. The variation trends of the four curves are almost consistent. The small discrepancies may be attributed to the disparity in the location of the mesh nodes. Thus, to balance the numerical accuracy and efficiency, 312024 cells were used in the following simulations.
The mathematical model was validated by comparing the industrial test data and simulation results from the perspectives of composition of generator gas at the outlet and dome average temperature, as shown in Table IV. This method has been used in many chemical reactors.[13,40,41] Although the model is developed based on industrial conditions, some extremely complex behaviors are simplified, so there are some small differences between simulation data and industrial data. But, in general, the simulated data have reached the requirement of industrial production. For example, the relative error of the dome average temperature is only 0.047 pct, and the proportion of CO and H2 reaches 83.3 pct, which can provide sufficient reductants for the reduction of ore in the shaft furnace. Therefore, the developed CFD model in this study is reliable and can be used to predict the transport phenomena in the dome zone of the COREX MG with PCI.
Effect of PCI in the Dome Zone on the Performance of the COREX MG
In this section, a detailed comparison of the velocity field, temperature field, gas composition distribution, and particle behavior in the dome zone of the COREX MG with single recycling dust injection (case 1) and dust-pulverized coal coinjection (case 2) are discussed.
Velocity field
Figure 5 shows the velocity field in the dome zone of cases 1 and 2. Both gas velocities show a uniformity and symmetry distribution. Figures 5(a) and (b) show that, in case 1, the positions of the burners are oppositely distributed, so after the high-speed fluid enters the dome zone through the burners, the gas collides at the center zone and causes a radial diffusion flow with a velocity of about 7 m/s. There is no obvious velocity gradient near the wall zone, which easily forms circulation and increases the residence time of particles, conducive to the complete combustion or gasification of dust particles. In case 2, with PCI in the dome zone, the pulverized coal particles are injected through the OCB, so the oxygen supply at the OCB is about 2.1 times that of the DB. Thus, the velocity in front of the OCB is much greater, as shown in Figure 5(c). In addition, the increase of the zone with high velocity, as shown in Figure 5(d), leads to the obvious expansion of the impact area between gas phases, which may affect the complex chemical reaction zone in the lower part of the dome.
A comparison of gas phase streamlines in the dome zone of cases 1 and 2 is shown in Figure 6. With the PCI in the dome zone, the local low-velocity circulation flow region in the upper part of the dome zone increases, which will make the movement of particles more complex and the residence time longer. Details about the particles’ behavior in the dome zone will be discussed in a later section. In addition, the streamlines along the height in the center region in case 2 are denser than those in case 1, indicating that the gas flow diffuses more fully after the impact between gas phases.
Temperature field
The temperature distribution in case 1 with single recycle dust injection is shown in Figure 7. The oxygen is blasted into the dome zone at high speed through the outer pipe of the DB, and then it is entrained with the surrounding dust particles and reducing gas, burning rapidly and forming a flame peak, with a maximum temperature of 2400 K. Figure 7(a) shows that the contour of the lower half of the flame peak is significantly smaller than that of the upper half. This phenomenon is mainly affected by the rising reducing gas from the packed bed of the COREX MG (which also can be regarded as the bottom boundary of the dome zone, as shown in Figure 1(b)). Previous works found that the initial momentum of the fuel jet and the buoyancy of the flame itself play an essential role in determining the flame envelope morphologic characteristics of a jet fire.[42,43,44] The relative angle between the momentum direction of the lower half of the flame peak and the buoyancy direction of the rising gas is an obtuse angle, which will hinder the development of the lower half of the flame peak. Also, the flame of the upward jet is observed to be much taller than that of the downward jet.[44] In addition, due to the release process of volatile in dust particles, there will be a low-temperature area with volatile enrichment in the interior of the flame peak, so the flame summit gradually develops in a ring shape. This phenomenon can be verified by the layered contour diagrams in front of the DB, as shown in Figure 7(b). The oxygen blasted through the OB is used to adjust the dome temperature, and its oxygen flow is low, so the temperature contour in front of the OB is smaller. There is no chemical reaction in the upper area of the dome zone, only affected by the plug flow. The temperature will be uniform and stable, finally maintaining at about 1330 K.
Figure 8 shows the temperature distribution in the dome zone with PCI. The temperature distribution in the dome zone changes significantly due to the redistribution of oxygen flow. Figure 8(a) shows that the high-temperature zone in front of the DB in case 2 is somewhat smaller than that in case 1. This is due to the decrease of oxygen volume at the DB in case 2. The flame structure is basically consistent with that in case 1. For the OCB, the oxygen is supplied through the inner pipe and a columnar high-temperature zone (about 2600 K) is formed, as shown in the red zone in Figure 8(b). In this high-temperature zone, the temperature first increases and then decreases. The main reason is that the pulverized coal particles and oxygen are, first, burned and a large amount of heat is released; then, the endothermic reactions, such as C(s)-CO2, C(s)-H2O, CO-H2O, and CH4-H2O, dominate. The temperature distribution is closely related to the chemical reaction. The transfer of the high-temperature zone indicates that after the PCI in the dome zone, the main reaction zone is transferred to the front of the OCB. Finally, the temperature of the generator gas is stable at about 1370 K. Compared with case 1, the dome average temperature in case 2 increases by about 40 K.
Gas composition
The composition of the generator gas at the outlet in the MG is directly related to the quality of the reduction gas used in the upper COREX shaft furnace. Figure 9 shows the gas distribution in case 1 on the vertical section of the DB. The volume fractions of CO and H2 are opposite to those of CO2 and H2O, respectively. Where the concentrations of CO and H2 are high, the concentrations of CO2 and H2O are low. In the front of the DB, the oxygen concentration is sufficient and the dust and the reducing gas rising from the packed bed have a fierce combustion reaction with O2, so the concentrations of CO2 and H2O are the highest. At the end of the gas stream, most of the oxygen is consumed. The secondary reactions of carbon residue (C(s)-CO2 and C(s)-H2O) are fully developed, and the concentration of CO increases rapidly, up to 85 pct. In the central zone of the dome, due to the limited combustion reaction and the diffusion effect of rising gas from the packed bed, the concentrations of CO and H2 are significantly higher than those in the surrounding area. With the increase of axial height, the space in the upper part of the dome expands and the concentration of each gas reaches equilibrium under the action of diffusion flow.
The volume fraction of the reducing gas (CO and H2) in case 2 is shown in Figure 10. It is obvious that the concentration distributions of CO2 and H2O are opposite to those of CO and H2, respectively, so the distributions of concentration of CO and H2, which are more important to the reduction processes in COREX, are discussed. As can be seen in Figure 10(a), due to the decrease in gas volume, the CO enrichment area in front of DB is closer to the furnace wall compared with that in case 1. In addition, affected by the gasification reaction near the OCB, there appears a region with a high concentration of H2 at the center of the DB vertical section, as shown in Figure 10(b). On the vertical section of the OCB, an H2 enrichment region similar to that in Figure 10(b) appears. This is because in the latter half of the flow, the oxygen concentration is low and multiple gasification reactions are fully developed, resulting in the generation of a large amount of H2.
Figure 11 shows the comparison of the proportion of reducing gas in the generator gas at the outlet of the dome zone. With a total of 150 kg/t coal injected into the dome zone, the proportion of CO2 in the reducing gas decreases by 7.8 pct and the volume fraction of H2 increases by 7.4 pct. The volume fraction of CO does not increase greatly. In total, the proportion of the effective component in generator gas increases by 10.9 pct. In addition to meeting the demand of the upper reduction process in the COREX shaft furnace, this hydrogen-rich reducing gas can also be blasted into a blast furnace to reduce the coke ratio.[45,46]
To further explore the effect of PCI in the dome zone on the concentration distribution of components, the variation tendencies of the concentration of CO and H2 along the central axis of cases 1 and 2 are compared, as shown in Figure 12. Due to the interaction of complex chemical reactions, the volume fractions of CO and H2 change significantly at the placement height of the burner. In case 1, dust combustion is the dominant process; thus, some rising reducing gas from the packed bed is burned to adjust the dome temperature. Therefore, the volume fraction of CO and H2 decreases sharply at the placement height of the burner. For case 2, the PCI in the dome zone is mainly to realize the coal gasification. The volume fraction of H2 at the placement height of the burner increases, while the volume fraction of CO decreases. The latter is consumed by the water–gas shift reaction. In addition, there are more complex gas components and reaction mechanisms in the dome after PCI, making the changes of CO and H2 concentration more obvious above the placement height of the burner. The generator gas outlet is located in the edge area of the dome, as shown in Figure 2, which is more vulnerable to the diffusion of reaction products than the central area of the dome. Therefore, there are some differences between the gas concentration at the top center and the data at the gas outlet.
Particle behavior
The trajectory and transformation behaviors of coal particles are very important for the stable operation of the dome zone. The burnout rate is an important index to evaluate the conversion extent of carbon in the particles, and it has been accepted by many scholars. It can be expressed as Eq. [13]:
where ma,0 is the ash content of the original dust and ma is the ash content of the burning residual.
Figure 13 shows the residence time and burnout rate of particles in cases 1 and 2. The trajectories of particles are consistent with the velocity field, indicating that the motion of particles is mainly affected by the gas flow. In case 1, the longest residence time of dust particles in the dome zone can reach about 70 seconds, while in case 2, the longest residence time of particles increased by about 10 seconds. Although the gas velocity increases in the dome zone, the aggravated circulation flow lengthens the residence time of particles. In addition, the simultaneous injection of pulverized coal and dust increases the number of particles, resulting in more complex movement behavior in the dome zone.
Figure 13(b) shows that the outer edge of the dust particle jet and surrounding oxygen form entrained flow, which can promote the complete combustion of dust particles. In case 2, the inner edge of the coal particle stream that is fully in contact with oxygen shows a better combustion behavior. Although there is some residual carbon in both particles, the burnout rate at the end of the jet is still nearly 100 pct. This also verifies that after oxygen depletion, the remaining carbon in the particles participates in the gasification reaction. In addition, the long residence time of dust and pulverized coal particles and the sufficient reaction space in the dome can also improve the conversion rate of both particles, which can finally reach more than 99 pct.
Conclusions
With the intent to gain a better understanding of the performance of a COREX MG for PCI, a 3-D mathematical model is developed to study the transport phenomenon in the dome zone of COREX MG with and without PCI. The comparison of the gas flow field, temperature distribution, gas composition, and particle trajectories is discussed. The following conclusions could be drawn from the present study.
-
1.
The gas flow field in the dome can be divided into a high-speed jet zone, impinging flow zone, diffusion flow zone, and circulation flow zone. In the case of PCI, the increase of high-speed jet beam leads to the expansion of impinging flow area.
-
2.
In the case of PCI in the dome zone, due to the redistribution of oxygen flow, the high-temperature zone in the dome zone is transferred, and the furnace top temperature increases by about 40 K.
-
3.
When 150 kg/t coal is injected into the dome zone, the proportion of CO2 in the reducing gas decreases by 7.8 pct, while the proportion of H2 increases by 7.4 pct. The total effective volume fraction of reducing gas in the outlet of MG can reach 83.3 pct.
-
4.
After PCI in the dome zone, the residence time of particles increases by about 10 seconds, which is caused by the aggravating circulation and the complexity of particle behavior. The burnout rate of dust and pulverized coal particles can reach more than 99 pct.
References
Q.A. Ali, U. Khayyam, and U. Nazar: J. Cleaner Prod., 2021, vol. 281, 124974.
A. Arasto, E. Tsupari, J. Kärki, J. Lilja, and M. Sihvonen: Int. J. Greenhouse Gas Control, 2014, vol. 30, pp. 140–47.
X. Hao, F. Shen, G. Du, Y. Shen, and Z. Xie: Steel Res. Int., 2005, vol. 76, pp. 694–99.
Y. Zhuo and Y. Shen: Appl. Energy, 2020, vol. 261, 114456.
F. Shen, B. Sundelin, K. Paulsson, E. Kapilashrami, G. Wei, M. Chu, and Y. Shen: Steel Res. Int., 2008, vol. 79, pp. 11–16.
M.A. Quader, S. Ahmed, S.Z. Dawal, and Y. Nukman: Renewable Sustain. Energy Rev., 2016, vol. 55, pp. 537–49.
X. Zhou, S. Pu, and N. Liu: IOP Con. Ser., 2020, vol. 768, p. 022003.
M.Y. Kou, H. Zhou, S.L. Wu, and Y.S. Shen: Arch. Metall. Mater., 2020, vol. 65, pp. 275–81.
K. Du, S. Wu, Z. Zhang, F. Chang, and X. Liu: ISIJ Int., 2014, vol. 54, pp. 2737–45.
D. Bi, Q. Guan, W. Xuan, and J. Zhang: Fuel, 2015, vol. 155, pp. 155–63.
W. Li, K. Li, X. Qu, R. Zhang, and J. Bi: Fuel Process. Technol., 2014, vol. 126, pp. 504–12.
R. Jovanović, B. Rašuo, P. Stefanović, D. Cvetinović, and B. Swiatkowski: Int. J. Heat Mass Transfer, 2013, vol. 58, pp. 654–62.
Z. Sun, Z. Dai, Z. Zhou, Q. Guo, and G. Yu: Ind. Eng. Chem. Res., 2012, vol. 51, pp. 2560–69.
Y.S. Shen, B.Y. Guo, A.B. Yu, P.R. Austin, and P. Zulli: Fuel, 2011, vol. 90, pp. 728–38.
Y. Liu, Z. Hu, and Y. Shen: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 2971–91.
Z. Zhou, Q. Xue, C. Li, G. Wang, X. She, and J. Wang: Appl. Therm. Eng., 2017, vol. 123, pp. 1096–1105.
Y.S. Shen, B.Y. Guo, A.B. Yu, and P. Zulli: Fuel, 2009, vol. 88, pp. 255–63.
M.P. Davis, R.J. Dry, and M.P. Schwarz: Proc. 3rd Int. Conf. on CFD in the Minerals and Process Industries, Dec. 2003, CSIRO, Canberra, Australia, pp. 305–12.
X. Yu and S. Shen: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2370–88.
Y. Liu and Y. Shen: Fuel, 2019, vol. 258, 116088.
S.C. Lee, M.K. Shin, S. Joo, and J.K. Yoon: Iron Steel Inst. Jpn. Int., 1999, vol. 39, pp. 319–28.
S.C. Lee, M.K. Shin, S. Joo, and J.K. Yoon: Iron Steel Inst. Jpn Int., 2000, vol. 40, pp. 1073–79.
S. Pal and A.K. Lahiri: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 103–14.
S. Pal and A.K. Lahiri: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 115–19.
F. Wang, C. Bai, Y. Yu, G. Qiu, and S. Zhang: Ironmak. Steelmak., 2009, vol. 36, pp. 590–96.
J. Sun, Z. Luo, and Z. Zou: Powder Technol., 2015, vol. 281, pp. 159–66.
Y. You, Z. Luo, Z. Zou, and R. Yang: Powder Technol., 2020, vol. 361, pp. 274–82.
K. Berger, C. Weiß, and W.L. Kepplinger: Steel Res. Int., 2008, vol. 79, pp. 579–85.
Q. Wang, J. Zhang, G. Wang, and H. Wang: Ironmak. Steelmak., 2020, vol. 47, pp. 344–50.
Y. Wang, H. Zuo, and J. Zhao: Ironmak. Steelmak., 2020, vol. 47, pp. 640–49.
B. Tian and S. Ji: Xin Jiang Iron Steel, 2018, vol. 24, pp. 37–39.
J. Xu, Q. Liang, R. Chen, B. Tian, Q. Zou, W. Yuan, and H. Liu: Sci. Sin. (Technol.), 2021, vol. 51, pp. 195–206.
Y. Liu and Y. Shen: Energy Fuels., 2018, vol. 32, pp. 4372–81.
G. Yu, J. Ni, Q. Liang, Q. Guo, and Z. Zhou: Ind. Eng. Chem. Res., 2009, vol. 48, pp. 10094–10103.
C. Zhou, Y. Wang, Q. Jin, Q. Chen, and Y. Zhou: J. Energy Inst., 2019, vol. 92, pp. 298–310.
Z. Liu, G. Wang, J. Zhang, J. Lee, H. Wang, M. Sun, and C. Wang: Energy Fuels, 2018, vol. 32, pp. 6155–66.
S.K. Ubhayakar, D.B. Stickler, C.W. Von Rosenberg, R.E. Gannon, C.W. Von Rosenberg, and R.E. Gannon: Symp. Combust., 1977, vol. 16, pp. 427–36.
Y. Shen, B. Guo, A. Yu, D. Maldonado, P. Austin, and P. Zulli: ISIJ Int., 2008, vol. 48, pp. 777–86.
H. Wang, J. Zhang, G. Wang, D. Zhao, J. Guo, and T. Song: Energies, 2017, vol. 10, pp. 255–66.
X. Zhang, Z. Luo, and Z. Zou: ISIJ Int., 2019, vol. 59, pp. 1972–81.
Y. Wu, J. Zhang, P. Smith, H. Zhang, C. Reid, J. Lv, and G. Yue: Energy Fuels, 2010, vol. 24, pp. 1156–63.
T. Mogi, H. Shiina, Y. Wada, and R. Dobashi: Fuel, 2011, vol. 90, pp. 2508–13.
D. Bradley, P.H. Gaskell, X.J. Gu, and A. Palacios: Combust. Flame, 2016, vol. 164, pp. 400–09.
S. Liu and L. Hu: Proc. Combust. Inst., 2019, vol. 37, pp. 3935–42.
H. Ahmed, D. Sideris, and B. Björkman: J. Mater. Res. Technol., 2020, vol. 9, pp. 16029–37.
H. Zhou, X. Tian, S. Yao, M. Kou, S. Wu, and Y. Shen: ISIJ Int., 2021, vol. 61, pp. 174–81.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (Grant Numbers: 51904023, 51804027), the Fundamental Research Funds for the Central UniversitiesQ10 (Grant Numbers: QNXM20210011, QNXM20210009) and the project of State Key Laboratory of Advanced Metallurgy (KF20-07) for their financial supports.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, H., Hu, Y., Tian, X. et al. Numerical Simulation of Pulverized Coal Injection in the Dome Zone of a COREX Melter Gasifier. Metall Mater Trans B 53, 3215–3228 (2022). https://doi.org/10.1007/s11663-022-02601-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02601-7