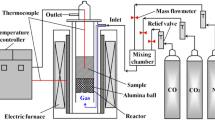
Abstract
A combined experimental and numerical analysis was conducted in this research on charging biochar composite briquette (BCB) to blast furnace (BF). The BCB used in this study was constituted of 10.10 wt pct carbon, 72.21 wt pct magnetite, 11.25 wt pct wustite, and 0.77 wt pct metallic iron. The reaction kinetics of BCB was determined using isothermal reaction tests under a simulated BF atmosphere. Numerical simulations were conducted to evaluate the BCB reaction behavior in BF and its effect on BF performance when 20 mass pct ore was substituted with BCB. The investigations revealed that, when ags = 1750 m2/m3, the predictions of the previously suggested composite briquette model accorded well with the experimental measurements. The findings of numerical simulations indicated that when the BCB was charged, it exhibited a rapid self-reduction in the temperature range of 600 °C to 900 °C (873 K to 1173 K). The BCB iron-oxide reduction and biochar gasification were completed above the cohesive zone. In the BCB charging operation, ore reduction was retarded in the solid temperature range less than 900 °C (1173 K), and it was prompted in the solid temperature range greater than 900 °C (1173 K). Local gas utilization increased in the mid-shaft and decreased at the burden surface. By charging BCB to BF, it was possible to increase BF productivity by 142 tHM/day, to decrease coke rate by 56.2 kg/tHM, to decrease PC rate by 8.9 kg/tHM, and to decrease BF CO2 emissions by 113 kg CO2/tHM.










Similar content being viewed by others
Change history
28 April 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11663-023-02792-7
Abbreviations
- A :
-
Specific area of solid burden (m2/m3)
- a gs :
-
Specific surface of iron-oxide particles in BCB (m2/m3)
- Cp:
-
Heat capacity, (J/kg/K)
- d :
-
Diameter, diameter of BCB (m)
- D, D eff :
-
Gas diffusivity, effective gas diffusivity (m2/s)
- E :
-
Enthalpy source (J/m3/s)
- f i :
-
Reaction fraction or biochar conversion of reaction i in Table 6 (–)
- H :
-
Total enthalpy (J/kg)
- ΔH i :
-
Reaction heat (J/kmol)
- h :
-
Heat transfer coefficient (W/m2/K)
- k i :
-
Reaction rate constant of reaction i in Table 6 (m/s, kg/s/atm)
- K i :
-
Equilibrium constant of reaction i in Table 6 (–)
- m :
-
Mass supply/consumption rate of the given element (kg/s)
- M :
-
Molar weight (kg/kmol)
- MSE:
-
Mean square error (–)
- P :
-
Pressure (Pa)
- Pr :
-
Prandtl number (–)
- R :
-
Gas constant (8.314 J/mol/K)
- Re :
-
Reynolds number (–)
- R i :
-
Chemical reaction rate of reaction i in Table 3 (kmol/m3/s)
- r :
-
Radial direction in BCB or BF (m)
- r i :
-
Chemical reaction rate of reaction i in Table 6 (mol/m3/s)
- S :
-
Source, units vary
- Sc :
-
Schmidt number (–)
- T :
-
Temperature (K)
- t :
-
Time (s)
- V cell :
-
Cell volume (m3)
- y :
-
Mass fraction (–)
- α :
-
BCB porosity (–)
- \(\varepsilon\) :
-
Volume fraction
- \(\xi\) :
-
Tortuosity factor in BCB (–)
- \(\phi ,\theta\) :
-
General dependent variable
- \({\Gamma }\) :
-
General diffusion coefficient
- ρ :
-
Density (kg/m3)
- η :
-
Liquid fraction (–)
- \(\lambda\) :
-
Thermal conductivity (W/m/K)
- µ :
-
Fluid viscosity (kg/m/s)
- \({\overset\rightharpoonup{\mathbf{U}}}_{{\text{g}}}\) :
-
Superficial gas velocity (m/s)
- \({\overset\rightharpoonup{\mathbf{V}}}_{{\text{s}}}\) :
-
Solid physical velocity (m/s)
- \({\overset\rightharpoonup{\mathbf{F}}}_{{\text{gs}}}\) :
-
Gas flow resistance (N/m3)
- 0:
-
Initial
- BCB:
-
BCB variable
- coke:
-
Coke variable
- ore:
-
Ore variable
- g:
-
Gas variable
- l:
-
Liquid variable
- s:
-
Solid variable
- e:
-
Environment variable
- Species or element name:
-
Variable of assigned species or element
References
World Steel Association: Steel Statistical Yearbook 2020 Concise Version, World Steel Association, Brussels, Belgium, 2020. https://www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook.html. Accessed 5 December 2021.
X. Zhu, H. Li, J. Chen, and F. Jiang: J. Clean. Prod., 2019, vol. 240, p. 118184.
X. Tan, H. Li, J. Guo, B. Gu, and Y. Zeng: J. Clean. Prod., 2019, vol. 222, pp. 823–34.
E. Mousa, C. Wang, J. Riesbeck, and M. Larsson: Renew. Sustain. Energy Rev., 2016, vol. 65, pp. 1247–66.
T. Norgate, N. Haque, M. Somerville, and S. Jahanshahi: ISIJ Int., 2012, vol. 52, pp. 1472–81.
L. Florentino-Madiedo, E. Díaz-Faes, and C. Barriocanal: Fuel Process. Technol., 2019, vol. 193, pp. 212–20.
S. Das, S. Sharma, and R. Choudhury: Energy., 2002, vol. 27, pp. 405–14.
M. Zandi, M. Martinez-Pacheco, and T. Fray: Miner. Eng., 2010, vol. 23, pp. 1139–45.
G. Jha and S. Soren: Renew. Sustain. Energy Rev., 2017, vol. 80, pp. 399–407.
J.G. Mathieson, H. Rogers, M.A. Somerville, and S. Jahanshahi: ISIJ Int., 2012, vol. 52, pp. 1489–96.
C. Wang, P. Mellin, J. Lövgren, L. Nilsson, W. Yang, H. Salman, A. Hultgren, and M. Larsson: Energy Convers. Manag., 2015, vol. 102, pp. 217–26.
Y. Liu and Y. Shen: Fuel Process. Technol., 2019, vol. 191, pp. 152–67.
H. Wang, M. Chu, W. Zhao, Z. Liu, and J. Tang: Metall. Mater. Trans. B., 2019, vol. 50B, pp. 324–36.
W. Zhao, M. Chu, Z. Liu, H. Wang, and Z. Ying: Metall. Mater. Trans. B., 2019, vol. 50B, pp. 1878–95.
H. Mizoguchi, H. Suzuki, and S. Hayashi: ISIJ Int., 2011, vol. 51, pp. 1247–54.
H. Yokoyama, K. Higuchi, T. Ito, and A. Oshio: ISIJ Int., 2012, vol. 52, pp. 2000–06.
M. Chu, Z. Liu, Z. Wang, and J.I. Yagi: Steel Res. Int., 2011, vol. 82, pp. 521–28.
H.M. Ahmed, N. Viswanathan, and B. Bjorkman: Steel Res. Int., 2014, vol. 85, pp. 293–306.
H. Wang, W. Zhao, M. Chu, Z. Liu, J. Tang, and Z. Ying: Powder Technol., 2018, vol. 328, pp. 318–28.
M. Chu, H. Nogami, and J.I. Yagi: ISIJ Int., 2004, vol. 44, pp. 510–17.
X. Yu and Y. Shen: Energy Fuels., 2019, vol. 33, pp. 11603–16.
S. Ueda, K. Watanabe, K. Yanagiya, R. Inoue, and T. Ariyama: ISIJ Int., 2009, vol. 49, pp. 1505–12.
E. Mousa, M. Lundgren, L.S. Ökvist, L.E. From, A. Robles, S. Höllsten, S. Bo, H. Friberg, and A. El-Tawil: J. Sustain. Metall., 2019, vol. 5, pp. 391–401.
M. Singh and B. Björkman: Ironmak. Steelmak., 2007, vol. 34, pp. 30–40.
Z. Yu, Z. Liu, H. Tang, and Q. Xue: Metall. Res. Technol., 2021, vol. 118, pp. 109–17.
H. Tang, Y. Sun, T. Rong, and Z. Guo: Powder Technol., 2021, vol. 377, pp. 832–42.
H. Tang, T. Rong, and K. Fan: ISIJ Int., 2019, vol. 59, pp. 810–19.
Z. Liu, Z. Yu, X. She, H. Tang, and Q. Xue: Metals., 2020, vol. 10, pp. 1666–75.
P.R. Austin, H. Nogami, and J.I. Yagi: ISIJ Int., 1997, vol. 37, pp. 458–67.
S.B. Kuanga, Z.Y. Li, D.L. Yan, Y.H. Qi, and A.B. Yu: Miner. Eng., 2014, vol. 63, pp. 45–56.
J.G. Mathieson, J.S. Truelove, and H. Rogers: Fuels., 2005, vol. 84, pp. 1229–37.
J. Chen, T. Akiyama, H. Nogami, J.I. Yagi, and H. Takahashi: ISIJ Int., 1993, vol. 33, pp. 664–71.
H. Tang, T. Qi, and Y. Qin: JOM., 2015, vol. 67, pp. 1956–65.
H. Tang, Z. Yun, X. Fu, and D. Shen: Metals., 2018, vol. 8, pp. 205–15.
H.X. Guo: Applied Chemical Kinetics, 1st ed. Chemical Engineering Press, Beijing, 2003, pp. 457–59.
CHAM: PHOENICS User Document, CHAM Ltd., London, 2000.
D.J. Gavel: Mater. Sci. Technol., 2017, vol. 33, pp. 381–87.
H. Zhang, L. Dong, H. Li, T. Fujita, S. Ohnishi, and Q. Tang: Energy Policy., 2013, vol. 61, pp. 1400–11.
Z. Zhou, X. Guo, G. Wang, Z. Xiang, and R. Wang: in Selected Papers of the 8th Annual Meeting of Chinese Iron and Steel Association, Metallurgical Industry Press, Beijing, 2011.
Acknowledgments
The authors thank the National Natural Science Foundation of China for supporting this work (Project No. U1960205).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11663-023-02792-7
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, H., Ma, L., Liu, Z. et al. RETRACTED ARTICLE: Charging Biochar Composite Briquette in Blast Furnace for Reducing CO2 Emissions: Combined Numerical and Experimental Investigations. Metall Mater Trans B 53, 2248–2261 (2022). https://doi.org/10.1007/s11663-022-02525-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02525-2