Abstract
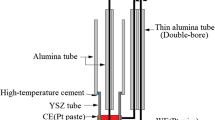
Neodymium oxide molten salt electrolysis in fluoride systems is the principal method of obtaining Nd and its alloys. However, the quality and performance of the Nd produced are limited by its carbon content, which is generated by the reaction of the anodic gas with Nd during the electrolytic process. In the present study, we investigated the mechanism and controlling factors of this reaction using a series of simulative experiments in which CO was pumped through a Ti tube at certain flow rates into a molten salt system containing a NdF3–LiF electrolyte and liquid Nd. The factors considered were the distance from the bottom of the Ti tube to the surface of the molten Nd (L), the temperature, and the electrolyte composition and viscosity. Our results demonstrate that the reaction between CO gas and Nd results in an increase in the carbon content of the Nd. Furthermore, we have established conditions that minimize this increasing carbon content, i.e., L = 35 mm in NdF3 85 pct–LiF 15 pct electrolyte at 1323 K. The side dissolution of Nd2O3 and Nd occurs in this electrolyte system, converting the initial NdF3–LiF electrolyte system into an NdF3–LiF–NdOF–NdF2 electrolyte system. Furthermore, our study revealed that the viscosity of the NdF3–LiF–(Nd2O3–NdF2) electrolyte system in the temperature from 1323 to 1373 K is between 0.010 and 0.040 Pa s, which is moderately low, and that the addition of Nd2O3 and NdF2 increases the viscosity of the NdF3–LiF electrolyte system.










Similar content being viewed by others
References
J.H. Bao: Mater. China., 2004, vol. 23, pp. 29–31.
Y.B. Liu, G.H. Chen, B. Yu, H.T. Huang, Q.J. Zhang, and W.C. Zhang: Chin. Rare Earths., 2021, vol. 42, pp. 133–43.
C.F. Liao, C. Guo, J.Y. Lin, B.Q. Cai, and X. Wang: Chin. Rare Earths., 2021, vol. 42, pp. 122–28.
X.L. Zhang, H.X. Liu, X. Zheng, W.H. Zhe, J. Liang, and Z. Jue: Adv. Mater. Res., 2013, vol. 800, pp. 496–500.
Y.T. Gong, H.J. Zhou, Q.S. Pang, and H.H. Wang: Rare Met. Cem. Carbides., 2018, vol. 46, pp. 1–5.
Y.F. Wu, G.L. Zhao, Z.X. Liu, Y.Q. Li, B.R. Guan, and Z.X. Liu: J. Chin. Soc. Rare Earths., 2017, vol. 35, pp. 606–13.
Y.T. Gong, Y.Z. Li, Q.S. Pang, Y.P. Xiong, and H. Zang: Chin. Rare Earths., 2020, vol. 41, pp. 133–43.
Q.S. Liu, W.D. Tang, and J.L. Wang: J. Chin. Soc. Rare Earths., 2015, vol. 33, pp. 737–46.
N.X. Feng, F.H. Liang, and C.S. Zhao: Nonferr. Met. Eng., 1998, vol. 50, pp. 60–64.
V. Constantin: Chin. J. Chem. Eng., 2015, vol. 23, pp. 722–26.
M.J. Sun, B.K. Li, L.M. Li, Q. Wang, J.P. Peng, Y.W. Wang, and C.P.C. Sherman: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 3161–73.
N. Xiong, Y. Tian, B. Yang, B.Q. Xu, T. Dai, and Y.N. Dai: Vacuum., 2019, vol. 160, pp. 213–25.
Y. Tian, B.Q. Xu, C.B. Yang, B. Yang, T. Qu, H.X. Liu, Y.N. Dai, and D.C. Liu: Metall. Mater. Trans. B., 2014, vol. 45B, pp. 1936–41.
L.H. Prentice, M.W. Nagle, T.R.D. Barton, S. Tassios, B.T. Kuan, P.J. Witt, and K.K. Constanti-Carey: The Minerals, Metals, and Materials Society, 2012, pp. 31–35.
J.Q. Xue, J. Zhang, W.B. Tang, Q. Bi, and Z.Y. Guo: Rare Met. Cem. Carbides., 2014, vol. 42, pp. 19–22.
F.J. Wu, J.Q. Ouyang, W.L. Yang, X. Luo, and X.Y. Wu: Hydrometall. China., 2017, vol. 36, pp. 430–33.
F. Li: Xi'an University of Architecture and Technology, Xi'an, 2018.
Y. Gao, Y.K. Shi, X.L. Liu, and B. Li: Electrochim. Acta., 2016, vol. 190, pp. 208–14.
Q.F. Huo: Metallurgical Industry Press, Beijing, 2002, p. 162.
G.X. Xu. Metallurgical Industry Press, Beijing, 1995, p. 156.
Z.F. Liu: Xi'an University of Architecture and Technology, Xi'an, 2015.
S. Feng, Y.F. Wu, Z.X. Liu, Y.F. Zhou, and S.N. Li: Chin. Rare Earths., 2021, vol. 42, pp. 1–11.
L.L. Liu, J.M. Sun, Z.P. Zhao, and J.G. Chen: Chem. Prod. Technol., 2008, vol. 15, pp. 59–61.
K.R. Liu, J.S. Chen, and X.J. Wei: Chin. J. Nonferr. Met., 2001, vol. 11, pp. 1118–21.
K.R. Liu, J.S. Chen, and X.J. Wei: Chin. Rare Earths., 2001, vol. 22, pp. 30–33.
X.L. Liu, C. Huang, and B. Li: Mater. Trans., 2017, vol. 58, pp. 395–99.
S.H. Seok, S.M. Jugn, Y.S. Lee, and D.J. Min: ISIJ Int., 2007, vol. 47, pp. 1090–96.
S. Sridhar, K.C. Mills, O.D.C. Afrange, H.P. Lörz, and R. Carli: Ironmak. Steelmak., 2000, vol. 27, pp. 238–42.
W.B. Xin, Y.C. Deng, Y.J. Jiang, and Y.Q. Wang: High Temp. Mater. Process., 2019, vol. 38, pp. 897–904.
Y.C. Deng, S.L. Wu, Y.J. Jiang, and S.Q. Jia: Mater. Metall. Mater. Trans. B., 2016, vol. 47B, pp. 2433–39.
Y.C. Deng, W.B. Xin, Y.J. Jiang, and C. Guo: Chin. Rare Earths., 2019, vol. 40, pp. 11–19.
Acknowledgments
The authors acknowledge financial support from the National Nature Science Foundation of China (Grant no. 52164035), the Natural Science Foundation of Inner Mongolia (Grant nos. 2018LH05018, 2020MS05009), and the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (Grant no. NJYT-20-B27).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., Xin, W., Jiang, Y. et al. Study on the Reaction Between Anodic Gas and Nd in Neodymium Oxide Molten Salt Electrolysis. Metall Mater Trans B 53, 1236–1243 (2022). https://doi.org/10.1007/s11663-022-02450-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02450-4