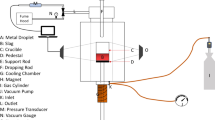
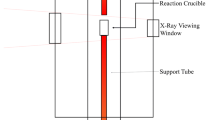
Abstract
An experimental study was performed to investigate the effect of ionic and electronic conductivity of oxidizing slags on the kinetics of decarburization of liquid metal droplets. An approach based on Wagner’s oxidation theory was developed to analyze the reaction kinetics with the variation of the slag conductivity. Despite the sufficiency of reactants, a sudden shutdown of decarburization reaction was observed for lower conductivity slag, whereas the reaction reached near thermodynamic equilibrium where the conductivity was higher. Based on this observation, a mechanism of accumulation of charge at the slag–metal interface has been proposed as the cause of premature shutdown of the reaction. While increasing basicity was also found to accelerate reaction kinetics and to eliminate or mitigate against premature shutdown of the reaction, it made no difference for slags of high electronic conductivity. This observation suggests that the desired rate of decarburization can be attained at lower basicity if the electronic conductivity of the slag is high.










Similar content being viewed by others
Abbreviations
- \({\left(\frac{\text{d}{n}_{\rm CO}}{\text{d}t}\right)}_{\text{peak}}\) :
-
Decarburization rate at the peak period (mol/m2-s)
- \({C}_{\text{FeO}}\) :
-
Concentration of FeO (mol/m3)
- \({E}^{\text{crit}}\) :
-
Electric field at the critical point of reaction shutdown (V/m)
- \({J}_{i}\) :
-
Flux of charged species i (mol/m2-s)
- \({a}_{\text{FeO}}\) :
-
Activity of FeO in the slag (–)
- \({a}_{i}^{\text{bulk}}\) and \({a}_{i}^{\text{int}}\) :
-
Activity of charged species i at the bulk slag and s/m interface respectively (–)
- \(\frac{{\text{d}}{n}_{\text{FeO}}}{{\text{d}}t}\) :
-
Flux of FeO in the slag (mol/m2-s)
- \(\frac{{\text{d}}{\mu }_{i}}{{\text{d}}x}\) :
-
Chemical potential gradient of species i (J/mol-m)
- \(\frac{{\text{d}}\varnothing }{{\text{d}}x}\) :
-
Electrical potential gradient (V/m)
- \({k}_{\text{s}}\) :
-
Mass transfer coefficient (m/s)
- \({{k}_{\rm s}^{\rm elec}}\) :
-
Electrochemical mass transfer coefficient (m/s)
- \({p}_{{\rm O}_{2}}\) :
-
Oxygen potential in the slag (Pa)
- \({t}_{i}\) :
-
Transference number of charged species i (–)
- \({z}_{i}\) :
-
Charge number of species i (–)
- \({\mu }_{i}\) :
-
Chemical potential of species i in slag
- \({\sigma }_{\rm el}\) :
-
Electronic conductivity of slag (S/m)
- \({\sigma }_{\rm ion}\) :
-
Ionic conductivity of slag (S/m)
- \({\sigma }_{\rm tot}\) :
-
Total conductivity of slag (S/m)
- \(\Delta x\) :
-
Boundary layer thickness in the slag (m)
- F :
-
Faraday constant (C/mol)
- R :
-
Universal gas constant (J/mol-K)
- T :
-
Temperature (K)
- \(V(t)\) :
-
Volume of droplet at time t (m3)
- y :
-
Ferric fraction (\(\frac{\text{Fe}^{3+}}{\text{Fe}^{2+}+\text{Fe}^{3+}}\)) in the slag (–)
References
G. Brooks, Y. Pan, and K.S. Coley: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 525–35.
Subagyo, G.A. Brooks, and K.S. Coley: Can. Metall. Q., 2005, vol. 44, pp. 119–30.
H. Sun, K. Gao, V. Sahajwalla, K. Mori, and R.D. Pehlke: ISIJ Int., 1999, vol. 39, pp. 1125–33.
K. Gao, V. Sahajwalla, H. Sun, C. Wheatley, and R. Dry: ISIJ Int., 2000, vol. 40, pp. 301–8.
M. Hayer and S.G. Whiteway: Can. Metall. Q., 1973, vol. 12, pp. 35–44.
P.G. Roddis: J. Iron Steel Inst., 1973, vol. 211, pp. 53–8.
J.B. See and N.A. Warner: J. Iron Steel Inst., 1973, vol. 211, pp. 44–52.
D. Sain and G.R. Belton: Metall. Trans. B., 1976, vol. 7B, pp. 235–44.
P.A.A. Distin, G.D.D. Hallett, and F.D. Richardson: J. Iron Steel Inst., 1968, vol. August, pp. 821–33.
H. Gaye and P.V. Riboud: Metall. Trans. B., 1977, vol. 8B, pp. 409–15.
C.L. Molloseau and R.J. Fruehan: Metall. Mater. Trans. B., 2002, vol. 33B, pp. 335–44.
D.J. Min and R.J. Fruehan: Metall. Trans. B., 1992, vol. 23B, pp. 29–37.
E. Chen and K.S. Coley: Ironmak. Steelmak., 2010, vol. 37, pp. 541–5.
D.E. Woolley and U.B. Pal: ISIJ Int., 1999, vol. 39, pp. 103–12.
G.G.K. Murthy, A. Hasham, and U.B. Pal: Ironmak. Steelmak., 1993, vol. 20, pp. 191–200.
G.G.K. Murthy, A. Hasham, and U.B. Pal: ISIJ Int., 1994, vol. 34, pp. 408–13.
S. Ramachandran, T.B. King, and N.J. Grant: J. Met., 1956, vol. 8, pp. 1549–58.
T. Gare and G.S.F. Hazeldean: Ironmak. Steelmak., 1981, vol. 8, pp. 169–81.
G.G.K. Murthy, Y. Sawada, and F. Elliot: J: Ironmak. Steelmak., 1993, vol. 20, pp. 179–200.
D.E. Woolley and U.B. Pal: Ironmak. Steelmak., 2002, vol. 29, pp. 125–32.
D.E. Woolley and U.B. Pal: Metall. Mater. Trans. B Process Metall. Mater. Process. Sci., 1999, vol. 30, pp. 877–89.
W.D. Judge, J. Paeng, and G. Azimi: Nat. Mater., 2021, https://doi.org/10.1038/s41563-021-01106-z.
W.D. Judge and G. Azimi: ECS Trans., 2018, vol. 85, pp. 91–102.
W.D. Judge, J. Paeng, and G. Azimi: Electrochim. Acta., 2021, vol. 389, p. 138755.
U. Pal, T. Debroy, and G. Simkovich: Metall. Trans. B., 1985, vol. 16B, pp. 77–82.
Y. Sayad-Yaghoubi, S. Sun, and S. Jahanshahi: in Molten Slags, Fluxes and Salts’97 Conference, 1997, pp. 839–44
X.J. Guo, R. Li, and R. Harris: Can. Metall. Q., 1999, vol. 38, pp. 33–41.
J.L. Speelman, W.F. Caley, and K.G. Leewis: Metall. Trans. B., 1989, vol. 20B, pp. 31–7.
Y. Sayadyaghoubi, S. Sun, and S. Jahanshahi: Metall. Mater. Trans. B., 1995, vol. 26B, pp. 795–802.
J. Biswas, K. Gu, and K.S. Coley: Metall. Mater. Trans. B., 2021, vol. 52B, pp. 3888–906.
J. Biswas, K. Gu, and K.S. Coley: Metall. Mater. Trans. B., 2021, vol. 52B, pp. 4215–29.
M. Barati and K.S. Coley: Metall. Mater. Trans. B., 2006, vol. 37B, pp. 51–60.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 2984–3001.
J. Berthon, A. Revcolevschi, and H. Morikawa: J. Cryst. Growth., 1979, vol. 47, pp. 736–8.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 2343–53.
H.-J. Engell and P. Vygen: Rep. Bunsen Soc. Phys. Chem., 1968, vol. 72, pp. 5–11.
M. Barati and K.S. Coley: Metall. Mater. Trans. B., 2006, vol. 37B, pp. 41–9.
W.R. Dickson and E.B. Dismukes: Trans. Metall. Soc. AIME., 1962, vol. 224, pp. 505–11.
M.T. Simnad, G. Derge, and I. George: J. Met., 1954, vol. 6, pp. 1386–90.
J.O.M. Bockris, J.A. Kitchener, S. Ignatowicz, and J.W. Tomlinson: Trans. Faraday Soc., 1952, vol. 48, pp. 75–91.
E.W. Mulholland, G.S.F. Hazeldean, and M. Davies: J. Iron Steel Inst., 1973, vol. 211, pp. 632–9.
M.D. Pomeroy: McMaster University, 2011.
Y. Sasaki, S. Hara, D.R. Gaskell, and G.R. Belton: Metall. Trans. B., 1984, vol. 15B, pp. 563–71.
M. Barati and K.S. Coley: Metall. Mater. Trans. B., 2006, vol. 37B, pp. 61–9.
C. Wagner: Z Phys. Chem., 1933, vol. B21, p. 25.
E.A. Pastukhov, O.A. Esin, and S.K. Chuchmarev: Sov. Electrochem., 1966, vol. 2, pp. 193–8.
Y. Takeda, S. Nakazawa, and A. Yazawa: Can. Metall. Q., 1980, vol. 19, pp. 297–305.
K.S. Goto, T. Kurahashi, and M. Sasabe: Metall. Trans. B., 1977, vol. 8B, pp. 523–8.
H. Larson and J. Chipman: J. Met., 1953, vol. 5, pp. 089–1096.
S. Wright and L. Zhang: in VII Int. Conf. on Molten Slags Fluxes and Salts, 2004, pp. 231–36.
M. Barati and K.S. Coley: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 169–78.
P.B. Drain, K. Gu, N. Dogan, R.J. Longbottom, M.W. Chapman, B.J. Monaghan, and K.S. Coley: ISIJ Int., 2021, vol. 61, pp. 734–44.
S. Jahanshahi: Imperial College of Science and Technology, London, 1980.
N. Simento, H. Lee, and P. Hayes: ISIJ Int., 1999, vol. 39, pp. 1217–23.
B. Sarma, A.W. Cramb, and R.J. Fruehan: Metall. Mater. Trans. B Process Metall. Mater., 1996, vol. 27B, pp. 717–30.
S. Ohguchi, D.G.C. Robertson, B. Deo, P. Grieveson, and J.H.E. Jeffes: Ironmak. Steelmak., 1984, vol. 11, pp. 202–13.
Acknowledgments
The authors are grateful to McMaster Steel Research Center and the Natural Sciences and Engineering Research Council for financial support. The authors wish to thank Dr. Kezhuan Gu for his valuable suggestions.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
The slag oxygen potential is a function of Fe3+/Fe2+ ratio and can be expressed as follows:
The slag conductivities can be expressed as a function of ferric fraction (y)
-
1.
Ionic conductivity
$${\sigma }_{\rm ion}={\sigma }_{0}-ay$$(A.3) -
2.
Electronic conductivity
$${\sigma }_{\rm e1}=b\left[\text{Fe}^{2+}\right]\left[\text{Fe}^{3+}\right]=by\left(1-y\right)$$(A.4)where the constants \({\sigma }_{0},\;a\) and b are functions of slag composition. From the Nernst–Einstein equation for ionic conductivity,
$${\sigma }_{0}=\frac{4{F}^{2}}{RT}\times ({C}_{\text{Ca}^{2+}}{D}_{\text{Ca}^{2+}}+{C}_{\rm Fe}{D}_{\text{Fe}^{2+}})$$(A.5)$$a=\frac{4{F}^{2}}{RT}{C}_{\rm Fe}{D}_{\text{Fe}^{2+}}$$(A.6)
From the diffusion-assisted charge hopping model for electronic conductivity,
Applying these to Eq. [3] and integrating from the slag–metal interface to bulk
We know from integration, \(\int \frac{\text{d}x}{{x}^{2}-{a}^{2}}=\frac{1}{2a}\text{ln}\frac{x-a}{x+a}+constant\)
Rights and permissions
About this article
Cite this article
Biswas, J., Coley, K.S. Decarburization of Iron Carbon Droplets with Oxidizing Slag: An Experimental Study to Understand the Effect of Ionic and Electronic Conductivity on Decarburization Kinetics. Metall Mater Trans B 53, 770–785 (2022). https://doi.org/10.1007/s11663-022-02448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02448-y