Abstract
A Cu-rich complex sulpfide concentrate (containing Sb as sulphosalts and gudmundite, and As as arsenopyrite) is roasted in Nitrogen atmosphere carrying traces of oxygen (\({\text{p}}^{{\text{O}}_{2}} \approx {10}^{-5.3}\text{ bar)}\). In situ measurements through QMS indicated that the volatilized species are mainly elemental sulfur, S2(g), and gaseous sulfur oxides. Sb- and As-bearing volatilized species could not be detected, owing to their low concentrations in the gas phase. Characterization studies through XRD and SEM-EDS confirmed that the condensates collected at room temperature during the roasting experiments comprised of (1) cyclo-octa sulfur, S8(s) and polysulfur oxides, Sn−xOx(s); (2) amorphous trisulfides of Sb and As; (3) and cubic crystalline trioxides of Sb and As. The solid phases in the condensate were found to be fine-sized (sub-micronic) and widely intermixed. Consequently, quantification of the solid phases in the condensates through direct measurement techniques like QEMSCAN was not possible. A novel approach of partial quantification of solid phases in the condensate through a stochastic model-based calculation approach is also presented. The model results suggested the occurrence of vapor-phase complexation of sulfides of Sb and As in the gas phase. Additional attributes of the volatilized species could be determined through a thermodynamic equilibrium calculation showing that the formation of the complex oxides, As4−nSbnO6(g), would be negligible compared to that of the complex sulfides, As4−nSbnS6(g).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The objectives of roasting the concentrates at a copper-making facility are drying and enrichment of incoming concentrate, partial oxidation of iron bearing phases, and volatilization of deleterious elements (such as Sb, As) for further pyrometallurgical extraction. In the recent times, the roasting unit became optional with the advent of continuous smelting and converting technologies such as Flash, Noranda, Isasmelt, and Ausmelt smelting. Nevertheless, the significance of roasting has again renewed, with the primary focus on volatilization of elements such as Sb and As, owing to the depletion of clean deposits and the increased production of complex concentrates.
There have been detailed studies evaluating the nature of Sb- and As-bearing volatilized and condensed species produced during roasting. The starting materials roasted in these studies were either pure compounds (As- and Sb-oxides and sulfides)[1,2,3,4] or dusts from non-ferrous pyrometallurgical processes, high in Sb and As contents.[5,6,7,8] These studies highlighted the attribute of vapor-phase complexation of volatile species of Sb and As leading to the formation of complex gaseous oxides, AsnSb4−nO6(g) and sulfides, AsnSb4−nS6(g), n = 1, 2, 3. Thermodynamic data for the vapor-phase complex oxides, AsnSb4−nO6(g) (n = 1, 2, 3), were determined by Li et al.[1] These calculations were based on the exceptional property of zero heat of disproportionation reactions of these mixed oxide compounds[9,10] and the results of vapor transport experiments using As2O3(s) and Sb2O3(s). However, for the sulfidic complexes, AsnSb4−nS6(g) (n = 1, 2, 3), thermodynamic data are available only for the species—AsSb3S6(g).[11] Equilibrium calculations based on these thermodynamic datasets supported the experimental findings that the formation of these complex species of As and Sb in the vapor-phase would facilitate an increased Sb-volatilization.[8,12]
Sb and As form non-volatile oxide compounds at the oxidation potentials \({\text{p}}^{{\text{O}}_{2}}>{10}^{-10}\text{ bar and }\,{{\text{p}}^{{\text{O}}_{2}}>{10}}^{-5.35}\text{ bar respectively}\).[13] Therefore, the volatilization of these elements from the sulfide concentrates during roasting have been studied in different controlled atmospheric environments.[14,15,16,17,18,19,20,21,22] However, the properties of volatilized and condensed Sb- and As-bearing species emanating from the sulfide concentrates during roasting have been rarely studied. Also, the effect of simultaneous release of volatile species by the other minerals such as pyrite present in the concentrate (comprising of labile sulfur[23]) on the gas phase complexation behavior of Sb and As has not been investigated. It is important to bridge this knowledge gap in order to improve the volatilization of As and Sb in industrial conditions during roasting, especially when the concentrate blends with increased proportions of complex concentrates are processed for the Cu extraction.
In this article, the condensates collected from the laboratory-scale roasting experiments performed by Prasad et al.[24] on polymetallic Cu-rich sulfide concentrates will be analyzed for the identification of condensed species. The experiments in this preceding study were carried out in Nitrogen atmosphere having traces of oxygen (O2 < 5 ppm, H2O < 5 ppm, \({\text{p}}^{{\text{O}}_{2}} \approx {10}^{-5.3}\text{ bar}\)), representative of feebly oxidizing conditions in the gas phase. They concluded through a low-temperature roasting experiment on a concentrate high in both Sb and As (considered high in As and Sb based on the smelter penalty limits[25]) that substantial Sb-volatilization takes place from the mineral gudmundite (FeSbS), owing to its decomposition between 300 °C and 400 °C into pyrrhotite and metallic-Sb. However, the mechanism activating Sb-volatilization from metallic-Sb was not elaborated. The current work attempts to further elucidate this Sb-volatilization process. Thermodynamic assessments are performed for (1) the stability of possible Sb- and As-bearing gaseous species during roasting and (2) the likelihood of Sb-As vapor-phase complexation in such conditions.
Additionally, this current study will portray certain limitations in the detection of Sb- and As-bearing volatilized species from in situ analyses of the gas phase through Quadrupole Mass Spectrometer during roasting of the sulfide concentrates. Despite these limitations, significant attributes of Sb- and As-bearing gaseous species will be gauged through a novel stochastic model-based calculation approach. This model will also facilitate a certain degree of quantification of the identified condensed phases in the condensate collected during a roasting experiment.
Materials
A Cu-rich polymetallic concentrate prepared by Minz et al.[26] from laboratory-scale flotation of crushed drilled core samples from the Rockliden deposit of Sweden was used in this study. The chemical composition of this concentrate is listed in Table I.
This concentrate is considered to be high in Sb and As based on the limits above which smelter penalties[25] are generally imposed. The As-bearing mineral in the concentrate is arsenopyrite (FeAsS) and the Sb-bearing minerals are tetrahedrite ((Cu,Ag,Fe,Zn)12Sb4S13), bournonite (CuPbSbS3), and gudmundite (FeSbS).[24]
The initial concentrate and the roasted calcines obtained from the laboratory-scale roasting experiments (to be described in the upcoming Section III–A) were analyzed for their chemical compositions by ALS Global AB, Luleå. The samples were melted in LiBO2 and digested in HNO3/HCl/HF. The measurements were made using ICP-SFMS in accordance with SS EN ISO 17294-2: 2016[28] and EPA method 200.8: 1994.[29]
Methods
Roasting Experiments in Vertical Tube Furnace
The Rockliden S7574 concentrate was tested in a vertical tube furnace setup described previously by Prasad et al.[24] The sample was introduced in the furnace and held in a cold chamber while the furnace heating was started. After the attainment of the desired furnace temperature, the sample was introduced in the hot zone. The furnace temperature was set higher than the aimed sample temperature, in accordance with the wall and center temperature relationship of this setup presented in a previous study by Prasad et al.[27] For instance, the furnace temperature set-point was 404 °C when the aimed sample temperature was 350 °C in the Experiment ID 350-10. A controlled gas atmosphere was maintained from the beginning by introducing Nitrogen (Chemical Nitrogen 4.6 from AGA-Linde, Purity ≥ 99.996 pct, H2O ≤ 5 ppm, O2 ≤ 5 ppm) as a carrier gas. Details of the experiments performed in this setup are listed in Table II.
Figure 1 presents a schematic of the vertical tube furnace used in this study. An optional water-cooled condensation plate was used in this apparatus for the Experiment IDs 700-1 and 350-10 to facilitate the partial condensation (at room temperature) of volatiles in the gas phase before leaving through the outlet. During the Experiment ID 350-10, the temperature was held at 350 °C for 10 hours, and a higher sample mass of 50 g was used to obtain sufficient amount of the condensates from this experiment for proper characterization.

Adapted from Prasad et al.[24]
Vertical tube furnace for roasting of concentrate.
TGA-DTA-QMS Study
The concentrate sample was also tested in a Netzsch STA 409 instrument (sensitivity ±1 μg) to determine the mass loss and phase transformations during heating through Thermo-Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTA), respectively. A Quadrupole Mass Spectrometer (QMS) connected to the thermo-gravimetric setup performed in situ analysis of the gas phase emanating from the sample. Quadstar 32-bit program was used to operate the mass spectrometer. Details of this equipment assembly are mentioned in Ahmed et al.[30] The experiments performed in this setup are listed in Table III.
The carrier gas used in these thermo-gravimetric experiments was again Chemical Nitrogen 4.6 (additionally containing H2O(g) ≤ 5 ppm, O2(g) ≤ 5 ppm). During the Experiment ID 1000-TG, the QMS was operated in Scanning Bargraph mode for the detection of gaseous species with mass-to-charge ratio (m/e) in a specified range between 1 and 300. In the Experiment ID 350-TG, the mode of QMS was Multiple Ion Detection (MID). In this mode, the ion current measurements in QMS were performed only for a set of preassigned m/e values.
Table IV presents the list of gaseous compounds intended for detection in the MID-mode of QMS. In case of the heteronuclear compounds of Sb and As, the m/e values were assigned for both molecular and ionic species (for instance, in case of As3SbO6(g), m/e = 442-443 for its molecular form, and m/e = 426-428 for its ionic form—\({\text{As}}_{3}{\text{Sb}}{\text{O}}_{5}^{+}\)[2]). This was to increase the possibility of the detection of these complex gaseous species during the in situ measurements in QMS. The Sb- and As-bearing compounds listed in Table IV were those which were predicted to form during the volatilization process by a thermodynamic model, presented later in this article (Section V). Additionally, the two complex sulfide species—As3SbS6(g) and As2Sb2S6(g), were included in this list based on the experimental evidences of their existence in the gas phase in the work of Mauser et al.[2] These two gaseous species were not estimated in the thermodynamic calculations due to unavailability of their thermodynamic data.
An important limitation of the QMS setup used in this work is that it cannot perform detection of species with m/e >510. Therefore, m/e values for the compounds with high molecular weights—AsSb3O6, As2Sb2S6, AsSb3S6, Sb4S3, and Sb4O6 could not be assigned for detection in the QMS (in the MID-mode) during the run of Experiment ID 350-TG (Table III).
SEM/EDS Analysis
Figure 2 shows the images of the condensate collected from the roasting Experiment ID 700-1 (Table II) in the vertical tube furnace, illustrating the physical appearance of the collected condensates from the roasting experiments in the vertical tube furnace. In both the Experiment IDs 700-1 and 350-10, the collected condensates had soft and polymeric texture. Each condensate was subsequently crushed manually in a mortar under liquid Nitrogen into a fine powder. A portion of the crushed powder was mixed with liquid epoxy, and the solidified epoxy mount was polished and carbon coated. These epoxy mounts were analyzed in a Zeiss Merlin FEG SEM (Scanning Electron Microscope) together with an Oxford Instruments X-Max EDS (Energy-Dispersive X-ray Spectroscopy) detector. The EDS was operated by AZtec software. A working distance of 8.5 mm, probe current of 500 pA, and accelerating voltage of 20 kV were employed for operating the SEM-EDS. Beam calibration in EDS was done using pure copper. The elemental composition obtained from EDS measurements is reported in mol pct after normalization, ensuring the summation of the concentrations for all identified elements to be 100 mol pct.
Images of the condensate collected from the Experiment ID 700-1 of roasting Rockliden S7574 concentrate in the vertical tube furnace. (a) Condensate as-deposited on the water-cooled Condensation plate (shown in Fig. 1); (b) Condensate peeled-off from the condensation plate for further investigations, having soft, polymeric texture
Powder XRD
The left over amount of powdered condensates after epoxy-molding were analyzed with XRD. These measurements were performed in a PANalytical Empyrean X-ray diffractometer in θ–θ geometry using Cu Kα radiation (λ = 0.154184 nm), beam current of 40 mA, 20 deg ≤ 2θ ≤ 90 deg with a step size of 0.026°/s, and beam voltage of 45 mV. A PIXcel 3D detector was used. A shallow sample holder with zero/negligible background was used to hold the condensate powders during the measurement, as the obtained quantities of the condensate powders were smaller than the normal sample size for the XRD-measurement. The obtained XRD patterns were evaluated in HighScore Plus (v4.7, a software from PANalytical B.V., Almelo, The Netherlands) using the FIZ-NIST ICSD (Inorganic Crystal Structure Database) database, version 2015-1.
Experimental Results and Discussion
Evaluation of Volatilization During Roasting
Results of the Experiment ID 1000-TG (Table III) in the TGA-DTA-QMS setup are presented in Figure 3. The highest ion current in QMS was measured for m/e = 32, representing O2(g). The nearly constant ion current for this m/e value over the entire heating cycle is also suggestive of it being the trace oxygen in the carrier Nitrogen gas. QMS detected a gaseous species with m/e = 64 as the predominant volatilized compound above 400 °C. m/e = 64 may represent either S2(g) or SO2(g), owing to their similarity in molecular weight (≈ 64). SO2(g) would evolve through the exothermic oxidation of sulfide minerals like chalcopyrite and pyrite. However, the peaks in the DTA curve corresponding to the QMS peaks for m/e = 64 are endothermic. Therefore, S2(g) with m/e = 64 is the predominant volatilized species, which is expected to be emanating from the pyrolysis (endothermic process) of chalcopyrite[31] and pyrite[23]. Simultaneous detection of SO(g) corresponding to m/e = 48 further suggests that the trace oxygen in the carrier Nitrogen partially oxidizes the S2(g). However, the QMS does not detect any Sb- or As-bearing gaseous species during this experimental run.
Figure 4 presents the molar quantities ΔS(mol), ΔSb(mol), and ΔAs(mol) of the elements volatilized during the roasting experiments on Rockliden S7574 concentrate in the vertical tube furnace at different temperatures between 200 °C and 700 °C (experimental details in Table II). Data in this plot are obtained from the chemical analyses and weights of the initial concentrate and the roasted calcines, presented in an earlier work by Prasad et al.[24] Evidently, at every temperature, the moles of S volatilized are approximately two order of magnitude higher than the quantities of Sb and As volatilized. This implies that the concentration of the volatilized Sb- and As-bearing gaseous species in the gas phase would be comparatively much lower than that of S2(g). Additionally, there will be a significant dilution owing to a constant flow of carrier gas (N2), thereby explaining the non-detection of Sb- and As- volatiles by the QMS (Figure 3) during the run of Experiment ID 1000-TG in the TGA-DTA-QMS setup.
Figure 4 in addition shows that noticeable Sb-volatilization started above 300 °C. Also, in the QMS measurements in Figure 3, S2(g) concentration in the gas phase was low below 400 °C. Based on these two observations, an experiment was planned in the TGA-QMS setup for heating the concentrate in Nitrogen atmosphere upto a temperature of 350 °C (midway between 300 °C and 400 °C), and holding at this temperature for 10 hours under a reduced Nitrogen flow of 50 mL/min (compared to 100 mL/min in the Experiment ID 1000-TG, Table III). The possibility of detection of Sb- and As-bearing phases in the QMS measurement was expected to increase due to likely low S2(g) volatilization at 350 °C.
Figure 5 presents the results of TGA and QMS measurements in the experiment at 350 °C (Experiment ID 350-TG in Table III). The QMS was set to measure ion currents for the selected m/e values ≤ 510 mentioned in Table IV. The concentrate experienced a total mass loss of approximately 0.8 pct in this experiment. The ion current measurements through QMS were in a wide range comprising of both positive and negative values. Consequently, a symmetric logarithmic scale developed by Webber[32] was used in Figure 5 (the transformation involved is \({{y}} = {\text{sgn}}\left({{x}}\right). {\text{log}}_{10}{(1+ |(x/C)|),}\) ‘sgn’ is the Signum function, C = 15.7) for the plotting of these widely scattered positive and negative ion current values. The QMS measurements could again clearly detect only O2(g) and the sulfur bearing S2(g) and SO(g) in the gas phase at this low temperature. The ion currents measured for rest of the m/e values corresponding to different Sb- and As-bearing species were in a narrow band of negative values below − 10-15 A. Tamura et al.[33] associated such negative ion current values to the offset error of ammeter in QMS, essentially implying non-detection. However, sporadic positive ion current values are also recorded for these m/e values in Figure 5, suggesting that the Sb- and As- bearing species did exist in the gas phase, but in very low concentrations. This experiment, therefore, demonstrated the limitations in the in situ detection of the Sb- and As-bearing volatilized species through QMS during the roasting of Cu-rich sulfide concentrates in inert atmosphere.
TGA-mass vs. time and temperature vs. time from TGA-DTA measurements (top graph); QMS measurements vs. time (lower graph) for heating Rockliden S7574 concentrate upto 350 °C and holding for 10 h in Nitrogen atmosphere containing traces of oxygen (H2O ≤ 5 ppm, O2 ≤ 5 ppm). Both the graphs having common x-axis of time (min). Data of QMS being in a wide range comprising of both negative and positive values are plotted in a symmetric logarithmic scale.[32] Only the QMS curves with consistent positive ion current measurements are labeled for the gaseous specie corresponding to the m/e value
Characterization of the Collected Condensates
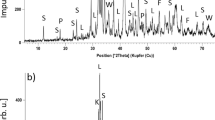
Figure 6 presents the XRD pattern of the condensate collected during the Experiment ID 700-1 (Table II) by roasting Rockliden S7574 concentrate at 700 °C in the vertical tube furnace. It shows that the condensate primarily comprises of elemental sulfur in the form of cyclo-octasulfur, S8(s).
Notably, similar to the failure in detecting Sb- and As-bearing species in the gas phase during measurement by the QMS, Sb and As compounds in the solid condensate could again not be identified through XRD. This was expected based on the fact that S-volatilization was two orders of magnitude higher than the quantities of Sb- and As-volatilization as shown in Figure 4.
XRD pattern of the condensate generated during the Experiment ID 350-10 of roasting the Rockliden S7574 concentrate at 350 °C is presented in Figure 7(a). It suggests that this condensate primarily comprises of Sb- and As-bearing compounds, unlike the elemental sulfur-rich condensate (Figure 6) from the roasting experiment at 700 °C. Secondly, the XRD pattern indicates that instead of the sulfides, expected to form during the roasting of sulfide concentrates in Nitrogen atmosphere, the condensate comprises of oxides. Most of the peaks in Figure 7(a) match with both As2O3(cubic) and Sb2O3(cubic) phases. Clearly, the traces of oxygen in the Nitrogen flow have contributed to the oxidation of Sb- and As-bearing volatiles. Figure 7(b) is the stem-plot showing only the peak intensities of the measured (> 5 pct) and reference patterns. Detailed examination of this stem-plot suggests that both in terms of peak positions and peak intensities, conformity of the measured pattern is relatively better with As2O3(cubic) compared to Sb2O3(cubic). The dominance of As2O3(cubic) over Sb2O3(cubic) in the 350 °C-condensate is perplexing since the Sb-volatilization was relatively much higher than the As-volatilization up to 400 °C in the roasting experiments, as shown in Figure 4.
(a) XRD pattern of the condensate obtained from roasting of Rockliden S7574 concentrate at 350 °C in Nitrogen atmosphere containing traces of oxygen. (b) Stem-plot showing only the peak intensities of the XRD pattern for the condensate (>5 pct), along with the peak intensities of pure compounds—As2O3(cubic) and Sb2O3(cubic). Data of the pure compounds from ICSD database
To further ascertain the constituting phases, the epoxy mounts of these condensates were examined in SEM-EDS. It was found through the EDS measurements that these condensates comprised of the elements—S, O, As and Sb. Figure 8 presents the EDS measurements of the elemental composition of the condensates performed at numerous spots over the polished surface of the epoxy mounts. The composition values in mol pct are arranged in the decreasing order of S-content along the x-axis. The nearly continuous nature of the plots, especially for the S-content in both the condensates indicates that the size of uniform phase fields in the condensate is much lower than the spot-size of EDS measurements. Otherwise, the measurement values for the elemental compositions would be clustered at the stoichiometric values corresponding to the specific compounds (for instance near 40 mol pct for the plot of As, if the EDS spot solely covers As2O3(cubic)). Therefore, the individual compounds in these condensates are fine-grained (in sub-micron sizes) and heterogeneously intermixed, evident from wide variation in the compositions over different spots.
EDS composition measurements at numerous spots for the condensates obtained from roasting Rockliden S7574 concentrate at (a) 700 °C (Experiment ID 700-1 in Table II), and at (b) 350 °C (Experiment ID 350-10 in Table II). The composition values are in mol pct. along the y-axes. The common x-axis represents the spot number. These numerous EDS spots are arranged along the x-axis in their decreasing order of S mol pct content
Following are the salient observations based on the elemental compositions in Figure 8(a) for the 700 °C-condensate:
-
a.
It comprises of low concentrations of Sb- and As-bearing phases in addition to the elemental sulfur, S8(s) suggested by the XRD pattern in Figure 6.
-
b.
There is substantial O-content of up to approx. 20 mol pct, much higher than the As- and Sb- contents. Also, the trend of O-content and S-content are opposite. These observations point towards the existence of solid sulfur-oxide phases in the condensate (possibly in the form of polysulfur oxides and peroxides[34]).
-
c.
The As- and Sb-contents increase with the increasing O-content, implying that these elements exist mainly as oxides in the condensate. Based on the findings from the XRD-measurement of the 350 °C-condensate in Figure 7, these oxide phases in the 700 °C-condensate are likely the compounds—As2O3(cubic) and Sb2O3(cubic).
-
d.
The Sb/As molar ratio is consistently very low at all the spots (Sb/As ≈ 0-0.2). However, as shown in Figure 4, the moles of Sb volatilized was approximately half of moles of As volatilized at 700 °C. This suggests that a significant amount of the volatilized Sb-phase could not be collected on the condensation plate. Notably, the trioxides of Sb and As condense at widely different temperatures. Li et al.[1] demonstrated this in their transpiration experiments where Sb2O3(cubic) condensed at approx. 550 °C and As2O3(cubic) condensed at approx. 220 °C. Coming back to the present study, the condensation plate being water–cooled and located far away from the hot zone (Figure 1) would be relatively more efficient in collecting As2O3(cubic) which condenses at a low temperature. Supposedly, during the roasting experiment, the Sb2O3(cubic) precipitated out of the gas phase at a higher temperature as fines suspended in the carrier gas. Most of these Sb2O3(cubic) fines apparently escaped from the furnace through the gas outlet (Figure 1) without depositing on the condensation plate, thereby resulting in a low Sb/As molar ratio in the 700 °C-condensate.
The prominent observations from the elemental compositions of the 350 °C-condensate shown in Figure 8(b) are as follows:
-
a.
In addition to the oxides confirmed from the XRD-measurement in Figure 7, the 350 °C-condensate also has sulfur-bearing phases in significant proportion. This is confirmed from its wide range of S-content between 7 and 85 mol pct over different EDS spot measurements.
-
b.
The O-content is low and nearly constant for S > 50 mol pct. In this range of S > 50 mol pct, the Sb and As contents increase with the decreasing S-content. These trends indicate that the elements Sb and As exist in the form of sulfides at these sites of high S-content (in addition to the trioxides confirmed from the XRD-measurement in Figure 7). The Sb- and As-sulfides are expected to be Sb2S3(s) and As2S3(s) based on the findings of Komorova et al.[35] and Hague.[36] The XRD-measurement shown in Figure 7 could not detect Sb2S3(s) and As2S3(s) in the 350 °C-condensate as these two compounds generally condense into their amorphous solid forms.[37,38]
-
c.
For S < 50 mol pct, O-content starts to increase, which is accompanied by a persistent increase in As-content, but there is a sudden drop in Sb-content to a low and nearly constant value. This behavior of low Sb/As molar ratio at significant levels of O-content (O > 10 mol pct) is common in both the 350 °C-condensate and the 700 °C-condensate. The more prominent drop of Sb/As molar ratio with increase in O-content in the 350 °C-condensate can again be attributed to the inefficient collection of Sb2O3(s) over the condensation plate (owing to the condensation of Sb2O3(s) starting from approx. 550 °C[1]).
Figure 9 presents the same compositional data for the 350 °C-condensate of Figure 8(b), but in the form of a scatter plot between O mol pct vs. S mol pct. EDS measurements on a hypothetical mixture containing finely intermixed As2S3(s), Sb2S3(s), As2O3(cubic), Sb2O3(cubic) would follow the line AB shown in this plot. The actual measurements follow this line as a lower limit of S-content. The measured data points above this line indicate that the spots contain elemental sulfur in addition to the trisulfides and trioxides of Sb and As. The upper limits of S-content also nearly fit over a straight line CD, almost parallel to the line AB. This line having a slope of − 1, suggests that the elemental sulfur, Sn(s) got partially oxidized to form polysulfur oxides, Sn−xOx(s) via an equal replacement of S atoms by O atoms (x-moles). Equal replacement of S by O means that, for instance, if the unoxidized elemental sulfur in the condensate was S8(s), the polysulfur oxide compounds would be S7O(s), S6O2(s) etc., and not S8O(s), S7O2(s) or S6O(s). Also, the S-content at O = 0 mol pct not reaching 100 mol pct yet again indicates that the elemental sulfur is fine-sized and much smaller than the spot-size of EDS measurements, such that no spot comprises of only the elemental sulfur.
Based on the above characterization investigations, the condensates collected at room temperature in this study during the roasting of Rockliden S7574 concentrate in Nitrogen atmosphere (bearing traces of oxygen) were found to consist of the following compounds:
-
1.
Elemental sulfur, Sn(s) and solid sulfur-oxides, Sn−xOx(s);
-
2.
Trioxides of Sb and As—Sb2O3(cubic), As2O3(cubic);
-
3.
Trisulfides of Sb and As—Sb2S3(s), As2S3(s)
Evaluation of Mineral Transformations Leading to Sb- and As-Volatilization at 350 °C
The Rockliden S7574 concentrate exhibited higher Sb-volatilization compared to As-volatilization below 500 °C in Nitrogen atmosphere, as presented in Figure 4. Arsenopyrite is the only As-bearing mineral in this concentrate.[24] Figure 10 presents a BSE image of a fully liberated particle of arsenopyrite in the calcine obtained from the Experiment ID 350-10 (Table II). The BSE gray-level throughout the particle is uniform, suggesting lack of any perceptible transformationin arsenopyrite at 350 °C. The elemental compositions at the indicated spots in this figure measured via EDS are presented in Table V. As content was found to be slightly lesser than in the theoretical stoichiometry of arsenopyrite (33.3 mol pct Fe, 33.3 mol pct As, 33.3 mol pct S). Therefore, arsenopyrite experienced only minor transformation at 350 °C, consequent to which the As-volatilization was low below 500 °C (Figure 4).
BSE image of a fully liberated particle of arsenopyrite in the calcine obtained from roasting Rockliden S7574 concentrate in Nitrogen atmosphere (containing traces of oxygen) at 350 °C via Experiment ID 350-10 (Table II). Sites of EDS spot measurements also indicated
Regarding substantial Sb-volatilization at low temperatures, Prasad et al.[24] in a previous study attributed this behavior to the thermal decomposition of gudmundite (FeSbS) into pyrrhotite and metallic-Sb between 300 °C and 400 °C. They also demonstrated that other Sb-bearing minerals in the concentrate did not experience any noticeable transformation at 350 °C, thereby concluding that the decomposition of gudmundite was the reason for a relatively high Sb-volatilization at low temperatures.
Further results from the evaluation of gudmundite transformation at 350 °C (Experiment ID 350-10, Table II) will now be presented. Interestingly, minor concentrations of As were found at the sites of partially transformed gudmundite as shown in Figure 11. Gudmundite in Rockliden S7574 did not contain any As from the beginning before roasting.[24] Figure 11 presents the EDS spectra of composition measurements at two different sites in the roasted calcine—one inside a fully liberated particle of gudmundite, and another in a particle hosting gudmundite-chalcopyrite intergrowth. As was detected in these spots of gudmundite in the roasted calcine at statistically significant levels, confirmed from low values of the statistical error, σ (corresponding to the As-content) in the displayed composition tables in Figure 11. (Composition for the site at the gudmundite-chalcopyrite intergrowth comprises additional elements—Cu, Zn, owing to small size of the uniform phase field in the complex intergrowth). Presence of As at these sites points towards a mechanism of Sb-volatilization which involves interaction of As-bearing species in the gas phase with the metallic-Sb generated from the thermal decomposition of gudmundite.[24] This observation is in line with the earlier evidences of formation of complex Sb-As compounds during volatilization when Sb and As coexist in the material undergoing roasting.[1,2,3,9,10,15]
EDS spectra for the composition measurement showing minor concentration of Arsenic at the sites of originally gudmundite (partially transformed) in the calcine obtained from roasting Rockliden S7574 concentrate at 350 °C (Experiment ID 350-10 in Table II) (a) in a fully liberated gudmundite particle and (b) in a particle hosting complex intergrowth of gudmundite and chalcopyrite
Thermodynamic Study
The Sb- and As-bearing volatilized species could not be consistently detected in the gas phase by QMS due to their low concentrations. Consequently, a thermodynamic calculation was performed for estimation of their quantities produced during the Experiment ID 350-10 (Table II). This was done in a thermochemical software FactSage 7.3,[39] using its ‘Equilib’ module and the FactPS database of pure substances. An important objective of this thermodynamic calculation was to compare the theoretical equilibrium concentrations of As- and Sb-bearing oxides and sulfides generated during roasting at 350 °C. Additionally, the intention was to investigate the possibility of formation of the Sb-As complex gaseous species. The data for the three known complex oxide compounds—AsnSb4−nO6(g) (n = 1, 2, 3), are available in the FactPS database. However, this database does not consist of the data for complex sulfide compounds AsnSb4−nS6(g) (n = 1, 2, 3). Among these three complex sulfides, thermodynamic data are available in the literature only for AsSb3S6(g) (Westphal[11] and HSC Chemistry[40]). Consequently, AsSb3S6(g) was included in the equilibrium calculation in FactSage by replicating its data from HSC chemistry. (Data of AsSb3S6(g) taken from HSC Chemistry into FactSage is presented in Appendix 1, Table A1.1).
Definition of Thermodynamic System and Initial Conditions
The calculations were primarily based on the findings from Prasad et al.[24] that Sb-volatilization at 350 °C occurred through the thermal decomposition of gudmundite (FeSbS) into pyrrhotite (Fe1−xS) and metallic-Sb. The thermodynamic framework, therefore, involved a set of simplified initial conditions of Sb(s) interacting with N2(g), O2(g), H2O(g) (components of the carrier gas), and S2(g) (labile sulfur volatilized from the concentrate) at 350 °C. An As-bearing sulfide vapor phase As4S4(g) was also included for consideration in the gas mixture. This was based on the detection of As at the sites of the transformed gudmundite, presented in Figure 11. The quantity of As4S4(g) was set as a study parameter and varied in a range of relatively insignificant values compared to the other gaseous components. The small quantities of As4S4(g) in the gas mixture were considered based on the observation of imperceptible transformation in the As-bearing mineral, arsenopyrite, at 350 °C (Figure 10, Table V). The model inputs of initial quantities of the reactants were calculated on the basis of 1 mole of Sb(s). Determination of the quantities of the gaseous compounds commensurate with 1 mole of Sb(s) is presented in Appendix 1, Table A1.2.
Results of the Thermodynamic Calculations and Discussion
Figure 12 presents the quantities of Sb- and As-bearing oxide and sulfide gaseous species (with available thermodynamic data) equilibrating at 350 °C corresponding to different initial quantities of As-sulfide in the gas phase.
Equilibrium quantities of gaseous oxide and sulfide compounds of Sb and As at 350 °C. Initial condition prior to equilibrium is 1 mole of Sb(s) together with a gas phase consisting of N2(g), O2(g), H2O(g), S2(g), and As4S4(g) in quantities listed in Appendix 1, Table A1.2
The results suggest that the concentration of individual oxide and sulfide compounds of As and Sb will be much higher than those of the complex heteronuclear Sb-As compounds—AsnSb4−nO6(g) and AsnSb4−nS6(g). However, the equilibrium calculation does not include the compounds—As3SbS6(g) and As2Sb2S6(g) due to unavailability of their thermodynamic data. These two compounds are expected to be present in much higher concentration than AsSb3S6(g) at lower temperatures, for instance at 300 °C as measured by Mauser.[2] Therefore, the vapor-phase complexation of sulfides of Sb and As might be prominent in reality, contrary to the indications from thermodynamic calculation.
Secondly, in Figure 12, the concentration of all the three heteronuclear oxide compounds—As3SbO6(g), As2Sb2O6(g), and AsSb3O6(g), are much lower than for the complex sulfide—AsSb3S6(g). Therefore, the Sb-As complex oxides are likely to be relatively insignificant in the gas phase compared to the Sb-As complex sulfides
Modeling of Condensate Compositional Domains
Phase quantification through direct measurements like QEMSCAN could not be possible for these condensates due to the small size of uniform phase fields, and the extensive heterogeneity in compositions over different spots (Figure 8). With focus on the 350 °C-condensate comprising substantial Sb- and As-bearing phases, an approach of partial quantification of solid phases in the condensate aided by a stochastic modeling approach is hereby presented.
The stochastic model calculates the elemental composition of the condensate by assuming random proportion of the identified solid phases—As2S3(s), Sb2S3(s), As2O3(s), Sb2O3(s), Sn(s), and Sn−xOx(s). Proportions of these solid phases in the condensate are assigned using random numbers between 0 and 1. The chemical stoichiometry of these individual solid phases are used subsequently to arrive at the elemental composition of the condensate in S, O, As, and Sb mol pct. A composition calculated by the model, therefore, tends to simulate the EDS measurement at a spot with non-uniform phase field, comprising varying proportions of the finely intermixed solid phases (in sub-micronic sizes, presented in Section IV–B).
The model is run multiple times with the same set of inputs (explanation of the model inputs forthcoming) to generate several distinct sets of random elemental composition. Since the condensate comprises of the four elements—S, O, As, and Sb, its composition can be plotted in six different binary scatter plots (4C2 = 6). Therefore, the scatter plots—S vs. O, S vs. As, S vs. Sb, O vs. As, O vs. Sb, and As vs. Sb are generated, and compositional limits/domains are obtained by bounding the plotted compositions. A compositional limit in S vs. O plot obtained by running the model with a specific set of input parameters is illustrated in Figure 13.
Model Input Parameters
The input parameters of the stochastic model are explained below:
-
1.
\(\left(\frac{{\text{Sb}}_{2}{{\text{S}}}_{3}\text{(s)}}{{\text{Sb}}_{2}{{\text{S}}}_{3}\text{(s)} + {\text{As}}_{2}{{\text{S}}}_{3}\text{(s)}}\right):\) Formation of the condensate took place through the condensation of volatiles produced during roasting. Therefore, the elemental composition of the condensate could show some correlations with the stoichiometry of the As- and Sb-bearing compounds in the gas phase. The thermodynamic analysis in Section V suggested that the Sb-As complex oxides, \({\text{As}}_n{{\text{Sb}}}_{{4-n}}{\text{O}}_{6}\left({\text{g}}\right)\) would be negligible during roasting. However, due to the unavailability of thermodynamic data for As3SbS6(g) and As2Sb2S6(g), the significance of the complex gaseous sulfides, \({\text{As}}_n{{\text{Sb}}}_{{4-n}}{\text{S}}_{6}\left({\text{g}}\right)\) could not be thermodynamically assessed. The stochastic model tests the possibility of Sb2S3(s) and As2S3(s) in the condensate to originate from the below condensation reaction, Eq. [1]:
$${2 }{\text{As}}_n{{\text{Sb}}}_{{4-n}}{\text{S}}_{6}\left({\text{g}}\right) \, \rightarrow \, n{\text{ As}}_{2}{{\text{S}}}_{3}\left({\text{s}}\right) \, + \, \left({4-n}\right) {\text{Sb}}_{2}{{\text{S}}}_{3}\left({\text{s}}\right);n = 1, 2, 3 $$(1)The model runs in two different modes as described in Table VI. When Mode = 1, it is considered that As2S3(s) and Sb2S3(s) in the condensate originated from the condensation of only the complex gaseous sulfide compounds—As3SbS6(g), As2Sb2S6(g), and AsSb3S6(g), in accordance with Eq. [1]. Consequently, the molar proportion of the trisulfides in the condensate, \(\left(\frac{{\text{Sb}}_{2}{{\text{S}}}_{3}\text{(s)}}{{\text{Sb}}_{2}{{\text{S}}}_{3}{(s)} + {\text{As}}_{2}{{\text{S}}}_{3}\text{(s)}}\right)\) at any site will be a particular value ϵ \(\left\{\frac{1}{4}, \frac{1}{2},\frac{3}{4}\right\}\) (as n ϵ {1,2,3} in Eq. [1]). A value from this set is selected randomly by the model in each run as presented in Table VI. Else, Mode = 2 considers the molar proportion of the trisulfides in the condensate to be a random value between 0 to 1, which would mean insignificance of the complex sulfide vapors \({\text{As}}_n{{\text{Sb}}}_{{4-n}}{\text{S}}_{6}\left({\text{g}}\right)\) in the gas phase.
Table VI Description of ‘Mode’ as an Input to the Stochastic Model for Condensate Composition -
2.
\({{R}}_{\text{O}}= 100 \times \left(\frac{{\text{Sb}}_{2}{{\text{O}}}_{3}\text{(s)}}{{\text{Sb}}_{2}{{\text{O}}}_{3}\text{(s) + }{\text{As}}_{2}{{\text{O}}}_{3}\text{(s)}}\right){, }{{R}}_{\text{O}} \, \in \, \left[{0, 100}\right]\): EDS measurements over the 350 °C-condensate in Figure 8(b) suggested a reasonably limited deposition of Sb2O3(s) on the condensation plate compared to As2O3(s). The model incorporates this effect through an input parameter, \({{R}}_{\text{O}}\) ϵ [0,100], signifying the relative inefficiency of the condensation plate in collecting Sb2O3(s) compared to As2O3(s). Molar ratio of the solid trioxides of Sb and As in the condensate would, therefore, be related to \({{R}}_{\text{O}}\) as per equation below:
$$\frac{{\text{Sb}}_{2}{{\text{O}}}_{3}\text{(s)}}{{\text{As}}_{2}{{\text{O}}}_{3}\text{(s)}}={\left(\frac{100}{{{R}}_{\text{O}}}- 1 \right)}^{-1}$$(2) -
3.
Smax: The condensate consists of sulfur in the form of Sn(s) and Sn−xOx(s), in addition to the sulfur in As2S3(s) and Sb2S3(s). Smax represents the maximum value of this extra sulfur content which can be found at any spot in the extensively heterogeneous condensate.
-
4.
Hmax: Regarding the polysulfur oxides, Sn−xOx(s) in the condensate, it is assumed that they were produced by the partial oxidation of condensed elemental sulfur, Sn(s) via Reaction [3], and not by the oxidation of gaseous elemental sulfur S2(g).
$${\text{S}}_n\left({\text{s}}\right)+\frac{{3x}}{{2}} \, {\text{O}}_{2}\left({\text{g}}\right)\rightarrow {\text{S}}_{{n-x}}{\text{O}}_{{x}}\left({\text{s}}\right) + x {\text{SO}}_{2}\left({\text{g}}\right)$$(3)Evidently, in the above reaction, it is also presumed that there is an equal replacement of S atoms by O atoms (x-moles) in Sn(s) to form solid sulfur oxides, SnOn−x (s). This is based on the slope of − 1 for the line CD representing sulfur upper limit in the O-vs.-S plot in Figure 9. Hmax signifies the oxygen content (mol pct) in the most oxidized polysulfur oxide phase in the heterogenous condensate. Oxygen content in Sn−xOx(s) is expressed as H, described in the below Eq. [4].
$$ H = \left(\frac{{x}}{n}\times 100 \right), H \in \, \left[0{H}^{\text{max}}\right]$$(4)For instance, if the condensate comprises of all of these polysulfur oxides—S7O(s), S6O2(s), S5O3(s), and S4O4(s) obtained from differing extents of oxidation of S8(s), Hmax should be equal to 50 mol pct, corresponding to the oxygen content in the most oxidized compound S4O4(s).
-
5.
Nrandom = Number of times the model is run repeatedly, generating a set of random elemental composition of the condensate: \(\left\{{\text{X}}_{\text{i}}{, i } \in \text{ As, Sb, S, O}\right\}\) in mol pct after each run. These composition values satisfy the below condition in Eq. [5].
$$\sum_{\text{i=As,Sb,S,O}}{\text{X}}_{\text{i}}= {100} \text{ pct}$$(5)
A detailed description of the mathematical formulation of the stochastic model is presented in Appendix 2.
Parametric Study
Appropriate values of the input parameters—Smax, Hmax, Mode, and \({{R}}_{\text{O}}\) are obtained by performing parametric analyses of the model and comparing the results with the EDS measurements of elemental composition for the 350 °C-condensate (already presented in Figures 8(b) and 9).
Figure 14 presents the parametric study results obtained by changing the values of Hmax and Smax in succession. The compositional limits in a S vs. O plot form domain boundaries in the shape of trapezium. Figure 14(a) shows that the value of Hmax decides the orientation of the line AD, one of the non-parallel sides in this trapezium (Lines AD1 to AD6 corresponding to increasing values of Hmax from 0 to 100 mol. pct). Superimposition of the EDS measurements suggests that the appropriate value of Hmax is 60 mol pct, corresponding to the line AD4, which offers the closest confinement of the data points from EDS measurements. Therefore, the most oxidized polysulfur oxide in the condensate should be approximately S3O5(s), owing to x ≈ 5 in the chemical formula, Sn−xOx(s) (rounded off from the exact value of x = 4.8, calculated by substituting H = Hmax = 60 mol pct and n = 8 corresponding to cyclo-octasulfur in Eq. [4]). Similarly, as shown in Figure 14(b), the location of the line CD is decided by the value of Smax and Smax = 75 mol pct leads to an apt validation with the EDS measurements. Consequently, the sulfur content representing elemental sulfur, Sn(s) (excluding sulfur in the As, Sb trisulfides), is below 75 mol pct at any site in the condensate.
Parametric analysis of the model performed by changing the input values for (a) Hmax and (b) Smax. The details of other input parameters are included in these plots of S vs. O elemental compositions. These plots show only the modeled compositional limits obtained by bounding modeled random composition data points (not shown in the plots). The plotted data points are from the data of EDS measurements for the 350 °C-condensate.
After determining the suitable values of Hmax and Smax, subsequently, the model is tested with changing input values of Mode and \({{R}}_{\text{O}}\). The effect of these changes are studied in the As vs. Sb plot owing to the higher relevance of Mode and \({{R}}_{\text{O}}\) in the modeled Sb- and As-contents. Figure 15(a) shows that changing the value of \({{R}}_{\text{O}}\) has no effect on the As-Sb compositional domain when Mode = 2 (Table VI, \(\left(\frac{{\text{Sb}}_{2}{{\text{S}}}_{3}\text{(s)}}{{\text{Sb}}_{2}{{\text{S}}}_{3}\text{(s) + }{\text{As}}_{2}{{\text{S}}}_{3}\text{(s)}}\right) \, \in \, \left[{0, 1}\right]\)). The same domain boundary-ABCDA is obtained irrespective of the different input values of \({{R}}_{\text{O}}\) = 0, 5, 10, 15, 20, 25, 40, 50, 75, 90, and 100 mol pct. Also, the superimposed EDS measurements suggest that the modeled compositional limits obtained with Mode = 2 are much wider than the scattered variations in the actual measurements. Interestingly, the modeled limits become responsive to the changes in \({{R}}_{\text{O}}\) when Mode = 1, as shown in Figure 15(b). The domain boundary obtained in each set of calculations is ApBpCqDqAp, where
-
p = 0, q = 0 to 5 for \({{R}}_{\text{O}}\) ≤ 75 mol pct; and
-
p = 1 and 2, q = 5 for \({{R}}_{\text{O}}\) > 75 mol pct.
Parametric analysis of the model by changing the input values of \({{R}}_{\text{O}}\) and (a) when Mode = 2, i.e., considering that Sb2S3(s) and As2S3(s) condensed from the individual gaseous sulfides of Sb and As; (b) when Mode = 1, i.e., considering that Sb2S3(s) and As2S3(s) condensed from complex sulfide vapors As4−nSbnS6(g). Also, the data of EDS measurements for the 350 °C-condensate superimposed.
Evident from Figure 15(b), the EDS measurements are well confined by the modeled domain boundary A0B0C2D2A0 (p = 0 and q = 2), corresponding to \({{R}}_{\text{O}}\) = 10 mol pct and Mode = 1. The agreement between the modeled composition limit and the EDS- in Figure 15(b) when Mode = 1 points towards the existence of the complex sulfide species, As4−nSbnS6(g) in the gas phase during volatilization of Sb and As from Rockliden S7574 concentrate at 350 °C. Additionally, it suggests \(\frac{{\text{Sb}}_{2}{{\text{O}}}_{3}\text{(s)}}{{\text{As}}_{2}{{\text{O}}}_{3}\text{(s)}} \approx \frac{1}{{9}}\) (ratio calculated by putting \({{R}}_{\text{O}}\) = 10 mol pct in Eq. [2]) in the 350 °C-condensate.
Validation of Parametrized Inputs
Table VII lists the values of the input variables of the stochastic model (described in Section VI–A) obtained through parametric analyses and subsequent comparison with the EDS measurements.
Until now, the S vs. O and As vs. Sb graphical representations have been used to demonstrate the close agreements of the modeled composition limits and the EDS measurements. Figure 16 presents further validation of the modeled composition limits in the other four possible binary scatter plot representations—Sb vs. S, As vs. S, Sb vs. O, and As vs. O. These illustrations prove the consistency of the modeled results generated from the parametrized inputs in all the possible graphical representations for the condensate composed of S, O, As, and Sb.
Modeled composition limits obtained by running the stochastic model with parameterized inputs listed in Table VII in different graphical representations (a) Sb vs. S, (b) As vs. S, (c) Sb vs. O, and (d) As vs. O. EDS measurements superimposed
Conclusions
Following conclusions can be drawn based on evaluation of the gas phase and solid-condensates collected during roasting of a Cu-rich complex sulfide concentrate (containing Sb in the form of sulphosalts and gudmundite, and As mainly as arsenopyrite) in Nitrogen atmosphere carrying traces of oxygen (\({\text{p}}^{{\text{O}}_{2}} \approx {10}^{-5.3}\text{ bar)}\):
-
1.
The condensate collected at room temperature comprises of the following phases:
-
a.
Elemental sulfur as cyclo-octa sulfur, S8(s); and polysulfur oxides, Sn−xOx(s)
-
b.
Amorphous trisulfides of Sb and As: Sb2S3(s) and As2S3(s)
-
c.
Cubic crystalline trioxides of Sb and As: Sb2O3(cubic) and As2O3(cubic).
-
a.
-
2.
Volatilization of Sb involves interaction of metallic-Sb(s) (produced by thermal decomposition of gudmundite) with vapors of As-sulfides in the gas phase.
-
3.
Thermodynamic analysis suggests that the Sb- and As-bearing compounds in the gas phase would mainly be their individual sulfides and oxides, and not their complex forms As4−nSbnX6(g) (X = O,S and n = 1, 2, 3). However, these thermodynamic assessments are not confirmatory for the complex sulfide phases (unlike for the complex oxides) owing to the lack of thermodynamic data for As3SbS6(g) and As2Sb2S6(g). The equilibrium concentration of AsSb3S6(g) (the only complex sulfide compound with available thermodynamic data) is much higher than those for all of the complex oxides—As4−nSbnO6(g) (n = 1, 2, 3).
-
4.
The solid phases in the condensate are fine-sized (sub-micronic) and widely intermixed leading to a heterogeneity in elemental composition of the condensates over different microscopic sites. Consequently, quantification of the solid phases in the condensates through direct measurement techniques like QEMSCAN is not possible.
-
5.
A stochastic model-based computation of compositional limits can facilitate partial quantification of the solid phases in the condensate. Also, the model results can facilitate vital assessments regarding the complexation of Sb- and As-gaseous species. The following attributes of the condensate collected at 350 °C could be determined from the model:
-
a.
The sulfur-content representing elemental sulfur, Sn(s) (excluding sulphur in the Sb and As-sulfides), is below 75 mol pct at any site in the condensate.
-
b.
The most oxidized polysulfur oxide in the condensate is S3O5(s).
-
c.
The compositional limits in As vs. Sb mol pct representation obtained from the model closely confine the plotted points from the numerous EDS measurements. These modeled composition limits were calculated by considering the formation of Sb2S3(s) and As2S3(s) in the condensate only through the condensation of the complex sulfide vapors, As4−nSbnS6(g) (n = 1, 2, 3). Therefore, the model results suggest the occurrence of vapor phase complexation of sulfides of Sb and As in the gas phase.
-
d.
The condensate collected at room temperature during roasting of the complex concentrate at 350 °C has a molar ratio of \(\frac{{\text{Sb}}_{2}{{\text{O}}}_{3}\text{(s)}}{{\text{As}}_{2}{{\text{O}}}_{3}\text{(s)}} \approx \frac{1}{{9}}\). The relatively low content of Sb2O3(s) compared to As2O3(s) can be related to high condensation temperature of Sb2O3(s) of approx. 550 °C as against approx. 220 °C for As2O3(s). By all accounts, most of the Sb2O3(s) escapes as suspended fines along with the gas phase.
-
a.
Abbreviations
- m/e :
-
Mass-to-charge ratio
- Wconc :
-
Weight of concentrate containing 1 mole of Sb in the form of gudmundite, g/mol-Sb
- \(n_{{\text{N}}_{2}\text{(g)}}^{\text{initial}}\) :
-
N2(g) introduced per mol of Sb(s) produced from gudmundite decomposition, mol/mol-Sb
- \(n_{{\text{O}}_{2}\text{(g)}}^{\text{initial}}\) :
-
O2(g) introduced per mol of Sb(s) produced from gudmundite decomposition, mol/mol-Sb
- \( n_{{{\text{H}}_{2} {\text{O(g)}}}} ^{{{\text{initial}}}} \) :
-
H2O(g) introduced per mol of Sb(s) produced from gudmundite decomposition, mol/mol-Sb
- \(n_{{\text{S}}_{2}\text{(g)}}^{\text{initial}}\) :
-
S2(g) introduced per mol of Sb(s) produced from gudmundite decomposition, mol/mol-Sb
- \(n_{{\text{As}}_{4}{{\text{S}}}_{4}\text{(g)}}^{\text{initial}}\) :
-
As4S4(g) introduced per mol of Sb(s) produced from gudmundite decomposition, mol/mol-Sb
- \(n_{\text{S}}^{\text{extra}}\) :
-
Sulphur content corresponding to Sn(s) and Sn−x Ox(s) in the condensate, mol pct
- \(n_{\text{O}}^{\text{extra}}\) :
-
Oxygen content corresponding to Sn−x Ox(s)in the condensate, mol pct
- \({\text{p}}_{{i=1,2,3}}\) :
-
Uniformly distributed random number ϵ [0, 1] generated at different stages in each run of calculation
- \({\text{p}}_{4}\) :
-
A randomly selected number from the set {0.25, 0.5, 0.75} when Mode = 1; a random number ϵ [0, 1] when Mode = 2
- \(n_{\text{S}}^{{\text{sul(As,Sb)}}}\) :
-
Sulphur content corresponding to As2S3(s) and Sb2S3(s) in the condensate, mol pct
- \(n_{\text{O}}^{\text{ox(As,Sb)}}\) :
-
Oxygen content corresponding to As2O3(s) and Sb2O3(s) in the condensate, mol pct
- \(n_{\text{Sb}}^{\text{sul}}\) :
-
Antimony content corresponding to Sb2S3(s) in the condensate, mol pct
- \(n_{\text{As}}^{\text{sul}}\) :
-
Arsenic content corresponding to As2S3(s) in the condensate, mol pct
- \(n_{\text{Sb}}^{\text{ox}}\) :
-
Antimony content corresponding to Sb2O3(s) in the condensate, mol pct
- \(n_{\text{As}}^{\text{ox}}\) :
-
Arsenic content corresponding to As2O3(s) in the condensate, mol pct
- \({\text{X}}_{\text{i=Sb,As,S,O}}\) :
-
Elemental composition of Sb, As, S, O calculated from the model in each run, mol pct
References
T. Li and J.P. Hager: in Reinhardt Schuhmann Int. Symp. Innovative Technol. React. Des. Extr. Metall., Proc. Symp., D.R. Gaskell, J.P. Hager, J.E. Hoffmann, and P.J. Mackey, eds., 1986, pp. 845–68.
J.E. Mauser, Metall. Trans. B 13, 511–513 (1982)
J.W. Hastie and J.P. Hager: in Elliott symp. proc., P. Koros and G. StPierre, eds., Iron Steel Soc., Warrendale, PA, 1990, pp. 301–24.
G.A. Brooks, W.J. Rankin, N.B. Gray, Metall. Mater. Trans. B 25B, 873–884 (1994)
D.P. Zhong, L. Li, C. Tan, Metall. Mater. Trans. B 48, 1308–1314 (2017)
L. Li, F. Wang, D. Zhong, C. Tan, Y. Yu, ISIJ Int. 57, 581–586 (2017)
M. Xu, L. Li, K.X. Mao, ACS Omega 4, 6968–6976 (2019)
C. Tan, L. Li, K. Li, D. Zhong, Sep. Purif. Technol. 194, 81–88 (2018)
J.H. Norman, H.G. Staley, J. Chem. Phys. 41, 1503–1504 (1964)
J. Drowart, S. Smoes, A. Vanderauwera-Mahieu, J. Chem. Thermodyn. 10, 453–464 (1978)
B. Westphal: Thesis, T-3778, Colorado School of Mines, 1989.
H. Jalkanen and E. Andersin: in Proc. Int. Terkel Rosenqvist Symp., S.E. Olsen and J.K. Tuset, eds., Norw. Inst. Technol., Trondheim, 1988, pp. 323–39.
S. Nakazaw, A. Yazawa, F.R.A. Jorgensen, Metall. Mater. Trans. B 30(3), 393–401 (1999)
G. Lindkvist and A. Holmstrom: in Adv. Sulfide Smelting, Proc. Int. Sulfide Smelting Symp. Extr. Process Metall. Meet. Metall. Soc. AIME, vol. 2, H.Y. Sohn, D.B. George, and Zunkel A D, eds., TMS-AIME, Warrendale, PA, 1983, pp. 451–73.
A.T. Jokilaakso, P.A. Taskinen, R.O. Suominen, K.R. Lilius, Scand. J. Metall. 23, 54–61 (1994)
W. Hong Lu and Z. lan Yin: Int. J. Miner. Process., 2016, vol. 153, pp. 1–7.
S. Shibuya, C. Tokoro, and T. Okura: in Proc. 7th Eur. Metall. Conf., 2013, pp. 111–22.
Å. Holmström: Ph.D. Thesis, R. Inst. Technol., Dep. Prod. Technol. (Stockholm), 1988.
K. Haga, B. Altansukh, A. Shibayama, Mater. Trans. 59, 1396–1403 (2018)
I. Wilkomirsky, F. Parada, A. Ulloa, A. Carrasso, and L. Jeraldo: in Proc. IV Int. Conf. Clean Technol. Min. Ind., vol. 2, Concepción Univ. Press, 1998, pp. 613–21.
R. Padilla and M.C. Ruiz: in Drying, Roasting, Calcining Miner., T. Battle, J. Downey, L. May, B. Davis, N. Neelameggham, S. Sanchez-Segado, and C. Pistorius, eds., Springer Int. Publishing, 2016, pp. 43–50.
I. Wilkomirsky, R. Parra, F. Parada, and E. Balladares: in COM 2014 - Conf. Metall. Proc., B. Davis, ed., Metall. Mater. Soc., Can. Inst. Min, Metall. Pet., 2014.
G. Majumdar, A.E. Morris, and M. Wadsley: Met. Extr.: Phase Stab. Diagrams, Ref. Module Mater. Sci. Mater. Eng., Elsevier, 2016.
P.N. Prasad, I. McElroy, A. Lennartsson, and C. Samuelsson: Metall. Mater. Trans. B, 2021, vol. 52, pp. 764–77.
P.R. Taylor and T.A.R. Putra: in Celebrating Megascale, Proc. Extr. Process. Div. Symp. Pyrometall., P.J. Mackey, E.J. Grimsey, R.T. Jones, and G.A. Brooks, eds., John Wiley & Sons, Inc., 2014, pp. 197–211.
F. Minz, N.-J. Bolin, P. Lamberg, C. Wanhainen, Miner. Eng. 52, 95–103 (2013)
P.N. Prasad, A. Lennartsson, C. Samuelsson, Metall. Mater. Trans. B 51, 1446–1459 (2020)
SS EN ISO 17294-2, Swed. Stand., Eur. Comm. Stand., 2016.
S.E. Long and T.D. Martin: EPA Method 200.8, U.S. Environ. Prot. Agency, 1994.
H.M. Ahmed, A. Persson, L.S. Ökvist, B. Björkman, ISIJ Int. 55(10), 2082–2089 (2015)
P.B.T.-P.M. BalÁž, ed.: in Extr. Metall. Act. Miner., vol. 10, Elsevier, 2000, pp. 95–142.
J.B.W. Webber, Meas. Sci. Technol. 24, 27001 (2012)
S. Tamura, H. Sugimoto, and M. Yashima: in AIP Conf. Proc., vol. 2132, 2019, pp. 190007:1-190007:12.
R. Steudel, F.C. Nachod and J.J. Zuckerman, eds., Engl., Walter de Gruyter, Berlin, 1977, p. 216.
L. Komorova, A. Holmstrom, I. Imris, Scand. J. Metall. 14, 103–112 (1985)
K.E. Haque: in Proc. Metall. Soc. Can. Inst. Min. Metall., R.S. Salter, D.M. Wyslouzil, and G.W. McDonald, eds., Elsevier, 1987, pp. 327–39.
L. Marton and C. Marton, eds.: in Adv. Electron. Electron Phys., Academic Press, New York, 1959, p. 379.
F.W. Glaze, D.H. Blackburn, J.S. Osmalov, D. Hubbard, and M.H. Black: J. Res. Natl. Bur. Stand. (1934)., 1957, vol. 59, pp. 83–92.
FactSage 7.3: © CRCT - Thermfact Inc. and GTT - Technologies.
HSC Chemistry 9: © Outotec Oy-Finland.
Acknowledgments
The authors thank (1) Swedish Strategic Innovation Program STRIM (a joint venture of Vinnova, Formas, and the Swedish Energy Agency) for the financial support; (2) personnel at Boliden Minerals AB for providing concentrate samples, chemical analyses, and QEMSCAN analyses; and (3) Ore Geology Division at Luleå Tekniska Universitet, Sweden for providing concentrate samples produced by them through laboratory-scale flotation in a previous study. The study is conducted within the context of CAMM2 (Center for Advanced Mining and Metallurgy) at Luleå University of Technology.
Funding
Open access funding provided by Lulea University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted November 12, 2020; accepted March 15, 2021.
Appendices
Appendix 1: Details of the Thermodynamic Calculation in FactSage
The thermodynamic data of AsSb3S6(g) taken from HSC Chemistry into FactSage is presented below in Table A1.1.
The basis of input quantities to the thermodynamic model was 1 mole of Sb(s). Table A1.2 below lists the quantities of the initial reactants in the thermodynamic system commensurate with 1 mole of Sb(s).
Appendix 2: Stochastic Model formulation
Stochastic model formulation depicting the relevance of input parameters and demonstrating the incorporation of random numbers p1, p2, p3 in the calculation. The model calculates elemental composition of the condensate (shown as a model output in the pie-chart on the right). Composition values are in mol pct
Figure A2-1 presents a schematic representation of the stochastic model formulation, depicting the calculation approach in each run of the model. The solid phase constituents of the condensate are designated the following individual segments in a pie-chart, representing their molar proportions in the 350 °C-condensate:
-
Segment 1—elemental sulphur (Sn) and solid polysulphur oxides(Sn−xOx)
-
Segment 2—the trisulfides, As2S3(s) and Sb2S3(s)
-
Segment 3—the trioxides, As2O3(cubic) and Sb2O3(cubic)
Based on the overall balance of elemental composition of the condensate in mol pct,
The extra sulphur (excluding S in Sb2S3(s) and As2S3(s)) can be between 0 to Smax mol pct in the condensate (its proportion in the condensate can be mathematically expressed as \({\mathrm{p}}_{1}\) Smax, as shown in Figure A2-1, where \({\mathrm{p}}_{1}\) ϵ [0, 1]). Secondly, any extra oxygen (excluding O in Sb2O3 and As2O3) would be part of solid sulphur oxides, Sn−xOx(s) formed via Reaction [3]. Since the model assumes equal substitution of S atoms by O atoms (based on the slope of − 1 for the line CD Figure 9), the sum of the molar quantities of Sn(s) and Sn−xOx(s) in the condensate will not change due to the occurrence of Reaction [3], Therefore,
Since the oxygen content in Sn−xOx(s) is between 0 to Hmax , its composition can be visualized in a subsidiary pie-chart elaborating the chemical composition of the constituents in Segment 1 in Figure A2-1 (PIP chart 1). The relationship can be mathematically expressed as
And using Eq. [A2-2],
Other than Sn(s) and Sn−xOx(s), the condensate has the trisulfides Sb2S3(s)-As2S3(s) and the oxides Sb2O3(s)-As2O3(s), which would bear the following stoichiometric relationships:
and,
The sum of the moles of trioxides and trisulfides of As and Sb would be \({(1-}{\text{p}}_{1}\) Smax). Also, the ratio of sulfide to oxide forms of As and Sb in the condensate can be random, i.e., \(\frac{{\text{p}}_{3}}{{(1-}{\text{p}}_{3}{)}}\), as specified in Segment 2 and Segment 3 in Figure A2-1. Accordingly, the total moles of trioxides will be \({\text{p}}_{3}{(1-}{\text{p}}_{1}\) Smax), and the total moles of trisulfides will be \((1-{\text{p}}_{3}{)(1-}{\text{p}}_{1}\) Smax). Subsequently, the following equations related to the quantities of elements in the trioxides and trisulfides can be derived using Eqs. [A2-2] to [A2-6].
The ratio of Sb2S3 to As2S3 in the condensate can be mathematically expressed as \(\frac{{\text{p}}_{4}}{{(1-}{\text{p}}_{4}\text{)}}\) by introducing another random number \({\text{p}}_{4}\). The assignment of \({\text{p}}_{4}\) is based on the value of the input parameter—Mode:
-
In case of Mode = 1, the trisulfides of Sb and As are considered to condense from their complex sulfide vapors, Asn−xSbxS6(g), such that \({\text{p}}_{4} \in \, \left\{\frac{1}{{4}}{, }\frac{1}{{2}}{,}\frac{3}{{4}}\right\}\) (Table VI). One value from this set is selected randomly in each calculation run (graphical representations of this case are in PIP-charts 2(b)-2(d) in Figure A2-1).
-
When Mode = 2, \({\text{p}}_{4}\) is a random number between 0 to 1 (PIP chart 2(a)), which implies that the condensation of trisulfides of Sb and As are not from the complex sulfide vapors, but instead from their individual sulfide vapor phases.
Accordingly, using Eq. [A2-7],
The proportion of Sb2O3 to As2O3 in the condensate is calculated based on the value of the input parameter \({{R}}_{\text{O}}\). Therefore, using Eq. [A2-9],
Using Eqs. [A2-4] to [A2-5], [A2-8], [A2-10] to [A2-14], the values of \(n_{\text{S}}^{\text{extra}}{, }n_{\text{O}}^{\text{extra}}{, }n_{\text{S}}^{\text{sul(As,Sb)}}{, }{n_{\text{O}}^{\text{ox(As,Sb)}}{, n}}_{\text{Sb}}^{\text{sul}}{, }n_{\text{As}}^{\text{sul}}{, }n_{\text{Sb}}^{\text{ox}}{, }n_{\text{As}}^{\text{ox}}\) are calculated. The moles of individual elements are calculated using the following equations:
Finally, the elemental composition in mol pct is derived using the below expression:
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prasad, P.N., Lennartsson, A. & Samuelsson, C. Volatilized and Condensed Sb- and As-Bearing Phases Produced During Roasting of Cu-Rich Complex Concentrate in Nitrogen Atmosphere with Oxygen in Traces. Metall Mater Trans B 52, 1866–1885 (2021). https://doi.org/10.1007/s11663-021-02154-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02154-1