Abstract
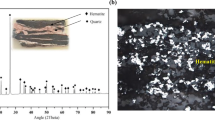
This study evaluates the carbothermal microwave reduction of low-grade banded hematite jasper iron ore for the preparation of potential feedstock for the alternative iron-making process. The coal sample act as transparent material to microwave irradiation at low temperature, however, above 600 °C, an exponential rise in temperature with a heating rate of ~ 220 °C/min was achieved. In contrast, charcoal showed an excellent response to microwaves at low temperatures. It was found that the iron-oxide reduction with coal is lean, resulting in lower metallization; however, the higher magnetite content in the concentrate improves the iron separation efficiency compared to charcoal. The separation efficiency of 67.3 pct is achieved at 6 pct C using coking coal compared to 44.9 pct with charcoal. It is inferred that the coal can be employed as a reductant for microwave carbothermal reduction. The ferrite balls with a yield of ~ 5 pct having 95 pct purity and iron-rich concentrate with ~ 87 emu/g magnetization and ~ 37 pct metallization with 73 pct yield are achieved with 18 pct charcoal in 15 minutes. The chemically bonded hematite and quartz phase enhances the electrical resistance and thus leading to efficient heating compared to the unbonded synthetic mixture. The fayalite formation creates localized melt zones that form’ hot-spots’ for microwaves and enhances the heating rate and, consequently, the reduction kinetics.











Similar content being viewed by others
References
1.K. Hara & M. Hayashi: J. Microw. Power Electromagn. Energy, 2011, 45, 137–147.
2.M, Hayashi, K. Takeda, K. Kashimura, T. Watanabe, & K. Nagata: ISIJ Int., 2013, 53, 1125–1130.
3.C.A. Pickles: Min. Eng., 2009, 22(13), 1112-1118.
4.J. Zhou, W. Xu, Z. You, Z. Wang, Y. Luo, L. Gao, C. Yin, R. Peng, & L. Lan: Scientific reports, 2016, 6, 25149.
5.J. Hunt, A. Ferrari, A. Lita, M. Crosswhite, B. Ashley, and A.E. Stiegman: J. Phys. Chem. C, 2013, 117, 26871–26880.
M. Stir, K. Ishizaki, S. Vaucher, & R. Nicula: J. Appl. Phys., 2009, 105.
7.K. Kashimura, K. Nagata, & M. Sato: Mater. Trans., 2010, 51, 1847–1853.
8.N. Standish and W. Huang: ISIJ Int., 1991, 31(3), 241-245.
9.A. Amini, K. Ohno, and T. Maeda: Scientific Reports, 2019, 8, 15023. https://doi.org/10.1038/s41598-018-33460-5.
10.K.E. Waters, N.A. Rowson, R.W. Greenwood, A.J. Williams: Sep. Purif. Technol., 2007, 46, 9–17.
11.S.W. Kingman and N.A. Roason: J Microwave Electromagnetic Energy, 2000, 35, 144-150.
S.J. Koleini and K. Barani: InTech, 2012, 79–104.
13.V.Rayapudi, S. Agrawal, and N. Dhawan: Min. Eng., 2019, 132, 202-210.
14.V. Rayapudi, S. Agrawal, and N. Dhawan: Pow. Technol., 2020, 362, 826-834.
15.A. Amini, K. Ohno, T. Maeda, & K. Kunitomo: Powder Technol., 2018, 338, 101–109.
16.H. Han, D. Duan, P. Yuan, & S. Chen: Ironmaking & Steelmaking, 2015, 42(7), 542-547.
B.A. Wills & J. Finch: (2015). Wills’ mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery. Butterworth-Heinemann.
18.K. Barani, S.J. Koleini, & B. Rezaei: Separation and Purification Technology, 2011, 76(3), 331-336.
19.A.W. Coats, & J.P. Redfern: Nature, 1964, 201(4914), 68-69.
20.O. Williams, A. Ure, L. Stevens, E. Binner, C. Dodds, S. Kingman, B. Das, P.S. Dash, and E. Lester: Energy & Fuel, 2019, 33(7), 6817-6828.
21.E. Binner, M. Mediero-Munoyerro, T. Huddle, S. Kingman, C. Dodds, G. Dimitrakis, J. Robinson, and E. Lester: Fuel Process. Technol., 2014, 125, 8−17.
22.E. Lester, S. Kingman, C. Dodds, and J. Patrick: Fuel, 2006, 85, 2057− 2063.
23.S. Mishra and G.G. Roy: Metall. Mater. Trans., 2016, 47(4), 2347-2356
Acknowledgments
The authors gratefully acknowledge the funding agency Science Engineering Research Board, New Delhi, India, for providing early-career research funds via ECR-000874/2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted January 28, 2020.
Rights and permissions
About this article
Cite this article
Agrawal, S., Dhawan, N. Microwave Carbothermic Reduction of Low-Grade Iron Ore. Metall Mater Trans B 51, 1576–1586 (2020). https://doi.org/10.1007/s11663-020-01883-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01883-z