Abstract
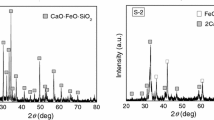
Vanishing of seaweeds in coastal areas has become a serious environmental problem. Part of the reason is nutrient deficiency in seawater, especially iron. Steelmaking slag is probably one of the best nutrient suppliers in terms of cost and quantity. Utilizing steelmaking slag to fertilize coastal seaweeds may simultaneously address the two challenges of seaweed restoration and byproduct treatment. However, direct usage of slag from a basic oxygen furnace is not practical because of negative effects such as a sharp pH increase and magnesium loss. To address the disadvantages, this work investigated the leaching behavior of a practical steelmaking slag in an artificial seawater and clarified the coupled effect of gluconic acid usage and slag carbonation on aiding the nutrient supply. Slag carbonation prevents a drastic pH increase and Mg loss, while gluconic acid induces formation of stable chelated complexes. The combination of slag carbonation and gluconic acid usage results in significant improvements in the nutrient supply. By leaching the slag, the maximum concentration of Fe in seawater increases to 0.29 mg L−1, 193 times larger than the value in the initial seawater (1.5 μg L−1).







Similar content being viewed by others
References
1 J.W. Fourqurean, C.M. Duarte, H. Kennedy, N. Marbà, M. Holmer, M.A. Mateo, E.T. Apostolaki, G.A. Kendrick, D. Krause-Jensen, K.J. McGlathery, and O. Serrano: Nat. Geosci., 2012, vol. 5, pp. 505–9.
2 E.M. Marzinelli, A.H. Campbell, A. Vergés, M.A. Coleman, B.P. Kelaher, and P.D. Steinberg: J. Appl. Phys., 2014, vol. 26, pp. 1089–96.
3 D.I. Walker and A.J. McComb: Mar. Pollut. Bull., 1992, vol. 25, pp. 191–5.
4 Y. Serisawa, Z. Imoto, T. Ishikawa, and M. Ohno: Fish. Sci., 2004, vol. 70, pp. 189–91.
5 Y. Suzuki, K. Kuma, I. Kudo, and K. Matsunaga: Phycologia, 1995, vol. 34, pp. 201–5.
K. Matsunaga, J. Nishioka, K. Kuma, K. Toya, and Y. Suzuki: Water Res. 1998, 32:3436-3442. 10.1016/S0043-1354(98)00113-4
7 M. Yamamoto, M. Fukushima, and D. Liu: Tetsu-to-Hagané, 2011, vol. 97, pp. 159–64.
8 M. Yamamoto, M. Fukushima, E. Kiso, T. Kato, M. Shibuya, S. Horiya, A. Nishida, K. Otsuka, and T. Komai: J. Chem. Eng. Jpn, 2010, vol. 43, pp. 627–34.
9 A. Blazevic, E. Orlowska, W. Kandioller, F. Jirsa, B.K. Keppler, M. Tafili-Kryeziu, W. Linert, R.F. Krachler, R. Krachler, and A. Rompel: Angew. Chem. Int. Ed., 2016, vol. 55, pp. 6417–22.
U.S. Geological Survey: Mineral Commodity Summaries 2018, 2018.
11 N. Ma and J.B. Houser: J. Clean. Prod., 2014, vol. 82, pp. 221–31.
12 X. Gao, M. Okubo, N. Maruoka, H. Shibata, T. Ito, and S.Y. Kitamura: Miner. Process. Extr. Metall., 2015, vol. 124, pp. 116–24.
13 K. Yokoyama, H. Kubo, K. Mori, H. Okada, S. Takeuchi, and T. Nagasaka: ISIJ Int., 2007, vol. 47, pp. 1541–8.
14 Y. Li and W. Dai: J. Clean. Prod., 2018, vol. 175, pp. 176–89.
15 H. Nakata, M. Yamaguchi, Y. Morinishi, and K. Masuda: Tetsu-to-Hagané, 2003, vol. 89, pp. 393–400.
16 T. Miki, T. Futatsuka, K. Shitogiden, T. Nagasaka, and M. Hino: ISIJ Int., 2004, vol. 44, pp. 762–9.
17 Y. Nakamura, A. Taniguchi, S. Okada, and M. Tokuda: ISIJ Int., 1998, vol. 38, pp. 390–8.
18 Y. Nakamura, T. Sato, K. Shitogiden, Y. Saito, H. Nakata, and A. Taniguchi: Tetsu-to-Hagané, 2003, vol. 89, pp. 438–45.
19 X. Zhang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2012, vol. 52, pp. 928–33.
20 X. Zhang, H. Atsumi, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2014, vol. 54, pp. 1443–9.
21 X. Zhang, H. Matsuura, and F. Tsukihashi: J. Sustain. Metll., 2015, vol. 1, pp. 134–43.
22 X. Zhang, H. Matsuura, and F. Tsukihashi: J. Sustain. Metll., 2016, vol. 2, pp. 123–32.
23 H. Matsuura, X. Zhang, L. Zang, G. Zhang, and F. Tsukihashi: Miner. Process. Extr. Metall., 2017, vol. 126, pp. 11–21.
24 Y. Lang, H. Matsuura, and F. Tsukihashi: J. Sustain. Metll., 2017, vol. 3, pp. 729–36.
25 W.M. Hayens, D.R. Ide, and T.J. Bruno, eds.: CRC Handbook of Chemistry and Physics, 97th Editi., CRC Press, Boca Raton, 2017.
L.H.N. Cooper: Proc. R. Soc. London Ser. B, 1937, vol. 124, pp. 299–307.
27 A. Hayashi, H. Tozawa, K. Shimada, K. Takahashi, R. Kaneko, F. Tsukihashi, R. Inoue, and T. Ariyama: ISIJ Int., 2011, vol. 51, pp. 1919–28.
28 M. Yamamoto and D. Liu: J. Mater. Cycles Waste Manag., 2013, vol. 15, pp. 264–8.
29 D.T. Sawyer: Chem. Rev., 1964, vol. 64, pp. 633–43.
30 W.J.J. Huijgen, G.J. Witkamp, and R.N.J. Comans: Environ. Sci. Technol., 2005, vol. 39, pp. 9676–82.
31 M. Pourbaix: Atlas of Electrochemical Equilibria in Aqueous Solutions, Second English Edition, National Association of Corrosion Engineers, Houston, 1974.
Chase Jr., M.W.: NIST-JANAF Themochemical Tables, Fourth Edition, 1998.
33 T. Misawa: Corros. Sci., 1973, vol. 13, pp. 659–76.
34 F.J. Prescott, J.K. Shaw, J.P. Bilello, and G.O. Cragwall: Ind. Engi. Chem., 1953, vol. 45, pp. 338–42.
35 T. Hamano, S. Fukagai, and F. Tsukihashi: ISIJ Int., 2006, vol. 46, pp. 490–5.
36 X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2009, vol. 49, pp. 1298–307.
Acknowledgment
The authors appreciate Prof. Hiroyuki Matsuura’s (The University of Tokyo) assistance with the experiment and helpful comments.
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 16, 2019.
Rights and permissions
About this article
Cite this article
Sakurai, Y., Yang, X., Hisaka, Y. et al. Nutrient Supply to Seawater from Steelmaking Slag: The Coupled Effect of Gluconic Acid Usage and Slag Carbonation. Metall Mater Trans B 51, 1039–1047 (2020). https://doi.org/10.1007/s11663-020-01805-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01805-z