Abstract
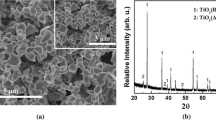
Direct reduction of TiO2 powder has been attempted for decades by researchers in an effort to decrease titanium (Ti) metal production costs. The main objective has been to avoid energy-intensive steps involved in production of primary Ti by the Kroll process. The emerging hydrogen-assisted magnesiothermic reduction process, which uses Mg to directly reduce TiO2 powder under a H2 atmosphere, has been shown to have the potential to compete directly with the Kroll process. The present studies represent an effort to understand the reduction reaction mechanisms of this process. Phase transformations and the reaction pathways are examined by SEM/EDX analysis of partially reduced powder cross-sectional, X-ray diffraction, and other analytical techniques. The results show important morphological changes, the prominent intermediate and final phases under the H2 atmosphere, as well as the local deposition behavior of the MgO byproduct. The effect of the specific surface areas of the initial particles is also discussed.
Similar content being viewed by others
References
ASM: in Titanium: A Technical Guide, 2nd edn., 2000, pp. 5–11.
R.R. Boyer: Jom, 2010, vol. 62, pp. 21–4.
J. Gambogi and S.J. Gerdemann: Titanium Metal: Extraction to Application, 1999.
US Patent 2,205,854: 1940.
D.J. Fray: Int. Mater. Rev., 2008, vol. 53, pp. 317–25.
Z.Z. Fang, J.D. Paramore, P. Sun, K.S.R. Chandran, Y. Zhang, Y. Xia, F. Cao, M. Koopman, and M. Free: Int. Mater. Rev., 2017, pp. 1–53.
E.H. Kraft: Summary of Emerging Titanium Cost Reduction Technologies, EHK Technologies for DOE/ORNL, Vancouver, WA, 2004.
Y. Zhang, Z.Z. Fang, P. Sun, S. Zheng, Y. Xia, and M. Free: JOM, 2017, vol. 69, pp. 1861–1868.
G.Z. Chen, D.J. Fray, and T.W. Farthing: Lett. to Nat., 2000, vol. 407, pp. 361–4.
D. Hu, A. Dolganov, M. Ma, B. Bhattacharya, M.T. Bishop, and G.Z. Chen: JOM, 2018, vol. 70, pp. 129–37.
I. Mellor, L. Grainger, R. Kartik, J. Deane, M. Conti, G. Doughty, and D. Vaughan: in Titanium Powder Production Via the Metalysis Process, 2015, pp. 51–67.
D.T.L. Alexander, C. Schwandt, and D.J. Fray: Acta Mater., 2006, vol. 54, pp. 2933–44.
C. Schwandt and D.J. Fray: Electrochim. Acta, 2005, vol. 51, pp. 66–76.
L.L. Benson, I. Mellor, and M. Jackson: J. Mater. Sci., 2016, vol. 51, pp. 4250–61.
M. Ma, D. Wang, W. Wang, X. Hu, X. Jin, and G.Z. Chen: J. Alloys Compd., 2006, vol. 420, pp. 37–45.
G. Crowley: Adv. Mater. Process., 2003, vol. 161, pp. 25–7.
W. Chen, Y. Yamamoto, and W.H. Peter: Key Eng. Mater., 2010, vol. 436, pp. 123–30.
X. Xu, P. Nash, and D. Mangabhai: JOM, 2017, vol. 69, pp. 770–5.
F.H. Froes: in A Historical Perspective of Titanium Powder Metallurgy, M. Qian and F.H. Froes, eds., Elsevier, Amsterdam, 2015, pp. 1–609.
F.H. Froes: JOM, 1998, vol. 9, pp. 41–3.
P. Waldner and G. Eriksson: Calphad Comput. Coupling Phase Diagrams Thermochem., 1999, vol. 23, pp. 189–218.
B.A. Borok, M.K. Rybal’chenko, I.A. Lavrent’ev, R.P. Shchegoleva, L.S. Golubeva, and V.F. Volobuev: Sov. Powder Metall. Met. Ceram., 1973, vol. 12, pp. 102–07.
T.H. Okabe, T. Oishi, and K. Ono: J. Alloys Compd., 1992, vol. 184, pp. 43–56.
T.H. Okabe, T. Oda, and Y. Mitsuda: J. Alloys Compd., 2004, vol. 364, pp. 156–63.
H. Zheng, H. Ito, and T.H. Okabe: Mater. Trans., 2007, vol. 48, pp. 2244–51.
T. Kikuchi, M. Yoshida, S. Matsuura, S. Natsui, E. Tsuji, H. Habazaki, and R.O. Suzuki: J. Phys. Chem. Solids, 2014, vol. 75, pp. 1041–8.
Y. Zhang, Z.Z. Fang, Y. Xia, Z. Huang, H. Lefler, T. Zhang, P. Sun, M.L. Free, and J. Guo: Chem. Eng. J., 2016, vol. 286, pp. 517–27.
Y. Zhang, Z.Z. Fang, P. Sun, T. Zhang, Y. Xia, C. Zhou, and Z. Huang: J. Am. Chem. Soc., 2016, vol. 138, pp. 6916–9.
K.T. Jacob and S. Gupta: J. Met., 2009, vol. 61, pp. 56–9.
K. Choi, H. Choi, and I. Sohn: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 922–32.
Y. Zhang, Z.Z. Fang, Y. Xia, P. Sun, B. Van Devener, M. Free, H. Lefler, and S. Zheng: Chem. Eng. J., 2017, vol. 308, pp. 299–310.
ASTM International: Standard Specification for Titanium Sponge: B299-13, 2015.
Y. Zhang, Z.Z. Fang, P. Sun, Y. Xia, M. Free, Z. Huang, H. Lefler, T. Zhang, and J. Guo: Chem. Eng. J., 2017, vol. 327, pp. 169–82.
Y. Xia, Z.Z. Fang, Y. Zhang, H. Lefler, T. Zhang, P. Sun, and Z. Huang: Mater. Trans., 2016, vol. 58, pp. 355–60.
S. Andersson, B. Collen, G. Kruuse, U. Kuylenstierna, A. Magneli, H. Pestmalis, and S. Asbrink: Acta Chem. Scand. II, 1957, vol. 11, pp. 1653–7.
B. Xu, H.Y. Sohn, Y. Mohassab, and Y. Lan: RSC Adv., 2016, vol. 6, pp. 79706–22.
Y. Le Page and P. Strobel: J. Solid State Chem., 1982, vol. 44, pp. 273–81.
A.C.M. Padilha, H. Raebiger, A.R. Rocha, and G.M. Dalpian: Sci. Reports - Nat., 2016, vol. 6, pp. 1–5.
R. Bhagat, D. Dye, S.L. Raghunathan, R.J. Talling, D. Inman, B.K. Jackson, K.K. Rao, and R.J. Dashwood: Acta Mater., 2010, vol. 58, pp. 5057–62.
K. Sreedhar and N.R. Pavaskar: Mater. Lett., 2002, vol. 53, pp. 452–5.
G. Yamaguchi and T. Tokuda: Bull. Chem. Soc. Jpn., 1967, vol. 40, pp. 843–51.
Y. Suzuki and Y. Shinoda: Sci. Technol. Adv. Mater., 2011, vol. 12, pp. 0–6.
B.A. Wechsler and R.B. Von Dreele: Acta Cryst., 1989, vol. B45, pp. 542–9.
M.S.R. Bolívar and P.B. Friedrich: in Procedings of EMC, 2009, pp. 1–17.
A. International: Phase Diagrams of Binary Titanium Alloys, Metals Park, 1987.
Acknowledgments
This work was financially supported by the U.S. Department of Energy (DOE), Advanced Research Projects Agency-Energy (ARPA-E), under contract number DE-AR0000420. The authors would also like to thank the corporate partners on the project, and the resources of the University of Utah. Additionally, a special thanks is extended to Material Data System (MDI) for their extension of an academic loan of Jade 2010 for XRD analysis, as well as KS Analytical Systems for helping to facilitate this arrangement.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted December 27, 2017.
Rights and permissions
About this article
Cite this article
Lefler, H., Fang, Z.Z., Zhang, Y. et al. Mechanisms of Hydrogen-Assisted Magnesiothermic Reduction of TiO2. Metall Mater Trans B 49, 2998–3006 (2018). https://doi.org/10.1007/s11663-018-1399-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1399-0