Abstract
The kinetics of aluminum removal from silicon melt to CaO-SiO2-Al2O3 slag was studied. A recently designed experimental setup using mechanical stirring was employed to focus the study on the chemical reaction. The slag and metal were found to reach chemical equilibrium in 300 seconds. A simple model could reproduce the experimental data satisfactorily. Both the experimental results and the model prediction further confirmed that the process was controlled by the chemical reaction, since the reaction rate constant was found to be independent of the amount of slag and the initial slag composition. The experimental data at equilibrium were compared with the model calculations. The discrepancy between the model calculations and the experimental data strongly suggests the need for careful thermodynamic measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicon is generally produced in large electric arc furnaces and tapped into ladles, where oxidative refining takes place to remove impurities such as Ca and Al. Current refining ladles are semiclosed on the top, lined with refractory, and equipped with bottom-blowing. Almost all companies worldwide use this technique. The last major improvement to oxidative refining took place a couple of decades ago with the introduction of the bottom plug. While bottom-blowing does help to improve the kinetic condition for refining, the process has still very poor mass transfer and lack of control of the heat balance. The poor kinetic conditions lead to an unnecessarily long process time, which in turn results in the loss of SiO2 to the surroundings and short life-time of refining ladle.
In order to develop a new type of refining vessel, a long-term project is currently being carried out in the present laboratory. To improve reaction rates, more efficient methods of agitation, such as mechanical stirring are considered. The facility of using mechanical stirring in the ladle to enhance the slag-metal reaction is one of the important topics of this project.
In many cases of slag-metal reactions, the rate limiting step is the mass transfer to and/or from the interface where the chemical reaction takes place rapidly. Consequently, most of the kinetic studies have addressed the mass transport of reactants to the interface and the product away from the interface without stirring. A number of studies have been carried out focusing on the boron and phosphorus transfer. Johnston and Barati[1] investigated the distribution of impurity elements between slag and MG-silicon doped with B and P at 1773 K (1500 °C) under argon atmosphere. The reaction time needed for the system to reach equilibrium was determined to be 2 hours. Nishimoto et al.[2] studied the removal of boron from molten silicon to CaO-SiO2 slag at 1823 K (1550 °C). It was found that the rate of boron removal was first-order and was controlled by mass transfer in the slag phase. Experimental data showed that equilibrium can be obtained within 2 hours.
Kinetic studies on aluminum removal from silicon are scarce. Weiss and Schwerdtfeger[3] investigated the equilibrium between silicon and CaO-SiO2-Al2O3 slag using silica crucibles. High-purity silicon and slag samples were held at 1773 K (1500 °C) under argon atmosphere for different periods of time and subsequently quenched. The aluminum content in silicon was found to increase with increasing alumina content in slag. It needed at least 3 hours for the system to reach equilibrium. These studies[1–3] indicate that the slag-metal reaction is a very slow process without stirring.
A few publications have investigated the effect of mechanical stirring as a means of enhancing slag-metal reactions.[4–6] White and Sichen[7,8] have recently studied slag-silicon reactions, wherein calcium is transferred from the slag to the liquid silicon. It has been found that the process is controlled purely by the chemical reaction when the rotation rate of stirring is sufficiently high. Further studies are needed to gain more insight into the kinetics of the process.
Kero et al.[9] investigated the refining characteristics of impurities in the industrial oxidative ladle refining process for MG-Si. The refining rates of elements were strongly correlated to the oxide basicity and correlated to equilibrium distribution of the element between slag and metal.
For some MG-silicon products, there may be relatively narrow specification windows for elements such as Al and Ca, challenging the refining process and our ability to control it. In order to have a stable process, meeting a narrow composition window, knowledge of both the kinetics and thermodynamics of the Si/slag system is essential. However, thermodynamic data relevant to the silicon refining process are still scarce; even the existing data are questionable with respect to their reliability. A typical example is the thermodynamic data for aluminum in silicon melt, particularly alumina-oxide activity data.
The activity coefficient of aluminum in silicon has been reported by Miki et al. based on the results using chemical equilibrium technique and Knudsen effusion method.[10,11] Ottem[12] has investigated the equilibrium between molten silicon and SiO2-CaO-Al2O3 slags. Dumay et al.[13] have measured the activities of aluminum and silicon by mass spectrometry. However, the reported data have shown certain inconsistency.
The aim of the present work was two-fold. The first purpose was to study the kinetics of the slag-metal reaction in the process of aluminum removal from liquid silicon to slag (CaO-SiO2-Al2O3). A kinetic model is developed to describe the reaction process. The second aim was to examine the reliabilities of the existing thermodynamic data using the slag-silicon equilibrium obtained in the experiments. This is essential to plan future thermodynamic measurements in the project.
Experimental
Material Preparation
A 90 wt pct Si-10 wt pct Al alloy was prepared by melting semiconductor-grade silicon (11N) and aluminum (3N) mixture in a graphite crucible under argon atmosphere at 1823 K (1550 °C) and kept at this temperature for 3 hours. The melt was rapidly quenched to room temperature and then saved in a desiccator before use. The composition of the Si-Al alloy was analyzed using inductively coupled plasma atomic emission spectroscopy. The master Si-Al alloy was added to semiconductor-grade silicon for preparing Al (1 wt pct)-Silicon samples.
Three different compositions of CaO-SiO2-Al2O3 slags were used in this study. CaO-SiO2 master slag (66 wt pct SiO2) was obtained by fusing high-purity silica and calcium oxide in a graphite crucible and subsequently granulated in water. CaO-SiO-10 wt pct Al2O3 and CaO-SiO-19 wt pct Al2O3 slags were prepared by fusing the mixture of additional Al2O3 powder and master slag in a graphite crucible under argon atmosphere at 1823 K (1550 °C) and kept at this temperature for 4 hours. The slag melt was rapidly quenched to room temperature and then saved in a desiccator before use. The slag compositions were analyzed by an atomic adsorption spectroscopy (AAS), using a Varian AA 280 FS instrument.
Setup and Procedure
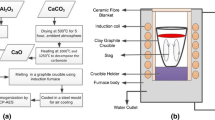
The experimental setup and procedure were described in detail in a previous publication.[7] A short description is provided here for convenience. The experimental setup is schematically shown in Figure 1. A vertical electrical tube furnace with an alumina tube as the reaction chamber was employed. The reaction tube was internally connected to a water-cooled quenching unit made of brass. The quenching chamber was equipped with two gas inlets, which introduced argon gas with high flow rate into the chamber for quenching the sample.
A graphite holder attached to a steel tube was used to support the working crucible. The steel tube was placed centrally from the top of the reaction chamber and was connected to a motor controlled lift. The lift could move the sample from the quenching chamber to the hot zone and from the hot zone to the quenched unit. The time to lift the sample for quenching was less than one second. A stirring motor was used to drive a graphite impeller that stirred the melt. The impeller was connected to the motor by a steel shaft kept in the steel tube (see Figure 1). The stirring motor was fastened to the lift. High-temperature-resistant rubber O-rings were used to seal the whole system.
The working crucibles and impellers were made of graphite. The impeller was 45 mm high and 15 mm wide. The mixing crucible was fabricated by boring four 18-mm diameter holes into a graphite blank, creating an internal volume with a quatrefoil profile. The resulting four vertical protrusions acted as baffles to generate vortex. In order to prevent the volatilization of SiO and other gaseous species from the crucible, the height of the crucible (110 mm) was twice of the expected height of the melt and a graphite lid was used above the crucible.
In a typical run, 40 g of 1 wt pct Al-Si was placed in the graphite crucible. The premelted slag in the form of lumps was placed on the top. The slag to silicon mass ratios were 1:1, 1:5 and 1:10. To start the experiment, the mixing crucible was placed in holding crucible and was mounted on the stainless steel supporting tube. The impeller blade was placed above the material in the mixing crucible. The reaction tube was then sealed, evacuated, and back-filled with argon. Argon gas with a constant flow rate (0.05 L/min) was passed through the reaction chamber throughout the whole experiment. The crucible assembly was kept in the quenching chamber until the run commenced. The furnace temperature was ramped up to 1823 K (1550 °C) at 2 to 3 K/min. After reaching the target temperature, the crucible assembly was lowered to a position in the reaction tube at about 1573 K (1300 °C) and kept there for 15 minutes. Preheating the crucible assembly was necessary to avoid thermal shock of the reaction tube. After this period, the crucible assembly was lowered all the way into the final position in the hot zone of the furnace. Preliminary experiments indicated that it took around 6 minutes to melt the slag and silicon. The impeller was inserted into the melt and positioned 10 mm above the bottom of the mixing crucible. The stirring motor was engaged immediately and the zero-time was noted. The previous work in this laboratory has evidently demonstrated that the rate of mass transfer is not improved at stirring speeds at and above 100 rpm.[8] Hence, in all the experiments a stirring speed of 100 rpm was employed. The stirring times ranged from 15 to 900 seconds. At the end of a run, the crucible assembly was rapidly lifted to the water-cooled quenching chamber using the lifting system. Immediately, a high flow of argon gas was introduced through the gas inlets in the quenching chamber to quench the sample. Thereafter, the crucible was taken out of the quenching chamber. The weight of the samples before and after each run were measured. Specimens were prepared for scanning electron microscopy (SEM) analysis. Pieces of slag and silicon were sent for chemical analysis.
Chemical Analysis
Chemical analyses of the samples were conducted at Elkem Technology analytical laboratory. Aluminum contents in silicon samples and compositions of slag samples were analyzed with an atomic adsorption spectroscopy (AAS), using a Varian AA 280 FS instrument. For the small slag samples, only aluminum contents in silicon were conducted because the amounts of slag were too small to make AAS analysis.
Result
Table I lists the conditions of all the experiments. The analyzed aluminum concentrations in silicon phase are included in table. Figure 2 shows the aluminum concentration in silicon phase and Al2O3 concentration in the slag phase as functions of time. The aluminum concentration decreases exponentially and levels out at about 120 seconds. No significant change in concentration is observed beyond 120 seconds of reaction time. The Al2O3 concentration in slag increases with time as aluminum in silicon is oxidized. The Al2O3 concentration in slag phase shows an increasing tendency in accordance with the variation of aluminum concentration in silicon phase. It appears that equilibrium between slag and metal is nearly reached at 300 seconds. The final concentrations of alumina in the slag samples analyzed by AAS are listed in Table II. Note that it is impossible to use AAS for the slags having very small mass.
It was found in previous work[7] that during initial period of stirring, significant emulsification takes place due to the low apparent interfacial tension. The agitation of the melt facilitates emulsification of the two phases and therefore enhances the slag-metal reaction. After 300 seconds, slag and metal have reached equilibrium. In this stage, the chemical driving force has become zero; only the interfacial tension plays an important role. As found in the previous work, no emulsification is observed when the slag and silicon are at equilibrium. A reliable chemical analysis demands the absence of any emulsification. To ensure the absence of emulsification, SEM analysis is carried out. Figure 3 shows SEM microphotograph and element mappings of slag-silicon interface after stirring time of 600 seconds. The SEM results evidently confirm the observation in the previous study.[7] It indicates that the chemical analyses are not affected by the entrainment of one phase into another.
(a) SEM micrograph and EDS mapping showing the slag-silicon phase boundary of Test No. 13 sample after stirring time of 600 s, (a) SEM micrograph. (b) SEM micrograph and EDS mapping showing the slag-silicon phase boundary of Test No. 13 sample after stirring time of 600 s, (b) Si mapping. (c) SEM micrograph and EDS mapping showing the slag-silicon phase boundary of Test No. 13 sample after stirring time of 600 s, (c) O mapping. (d) SEM micrograph and EDS mapping showing the slag-silicon phase boundary of Test No. 13 sample after stirring time of 600 s, (d) Al mapping
Discussion
Effect of Initial Slag Composition
Figure 4 shows the concentration of aluminum in the silicon phase as a function of time in the cases of different initial Al2O3 contents in the slag phase. In all these experiments, a slag-metal mass ratio of 1 (40/40g) is employed. It is seen that the final Al content in the silicon phase increases with the increase of the initial Al2O3 content in slag. Although the aluminum in the silicon would react with CaO to some extent, the main reaction for the transfer of aluminum to slag is due to Reaction [1],
At equilibrium, the following relationship is maintained:
where K 1 is the equilibrium constant of Reaction [1] and a i stands for activity of component i (Si, Al, SiO2, and Al2O3). Considering the difference in the initial content of Al2O3, Eq. [2] can be rewritten as
where \( \gamma_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} \) is the activity coefficient of Al2O3, \( {\text{X}}_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }}^{ 0} \) and \( {{\Delta }}X_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} \) are the initial mole fractions of Al2O3 and the change of mole fraction of Al2O3 due to the reaction, \( {\text{f}}_{\text{Al}} \) is the activity coefficient of Al in silicon, \( ({\text{mass pct Al}})^{0}, \) and \( {{\Delta (\text{mass\; pct\; Al})}} \) are the initial concentration of Al and the change of the concentration of Al due to the reaction. The change of total moles of slag is relatively small considering the initial amount of slag. The weight loss of the sample (slag + Si) was only 0.07 g, which corresponds to less than 0.1 pct of the total weight. The extremely small weight loss indicates that the effect of volatilization on the slag composition is negligible. Therefore, \( {{\Delta ({\rm mass pct Al})}} \) is related to \( {{\Delta }}X_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} \)through Eq. [3]
where \( M_{\text{Al}} \) is molecular weight of aluminum, \( n_{\text{total}}^{ 0} \) is total moles of initial slag, and \( m_{\text{metal}}^{0} \) is initial mass of metal phase. To keep K 1 constant, a higher \( X_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }}^{ 0} \) would lead to a smaller \( {{\Delta }}X_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} \) and therefore smaller \( {{\Delta (mass pct Al)}} \) as shown in Figure 4. The results show evidently that reducing the initial content of Al2O3 in the slag would benefit greatly the aluminum removal.
Effect of Amount of Slag
Figure 5 presents the concentrations of aluminum in the silicon phase as functions of time for different amounts of slag. In all these experiments, an initial slag composition of 34 wt pct CaO-66 wt pct SiO2 are employed and the experimental temperature is 1823 K (1550 °C). The less amount of slag also leads to higher final Al concentration in silicon. Inserting Eq. [3] into Eq. [2′] leads to
Equations [3] and [2″] indicate that larger amount of slag (\( {\text{larger}}\;n_{\text{total}}^{0} \)) leads to higher \( {{\Delta (\text{mass pct Al})}}\) in the final stage. The experimental results show the strong effect of the amount of slag on the final aluminum concentration in the silicon. In industrial practice, an optimization should be made considering (1) the target of the concentration of Al in the final product, (2) the viscosity and other physical properties of the slag based on the composition, and (3) the cost of the raw materials.
Kinetics of the Reaction Process
In the previous work,[8] a similar system using identical experimental setup was studied. It was found that the reaction was solely controlled by chemical reaction when the rotation rate reached 100 RPM, the mass transfer being so fast because of the mechanical stirring. It would be interesting to model the reaction process, as it would provide a basis for applying this approach to industrial practice. It was found that slag-metal emulsified together at the stages when the reaction was not in equilibrium and separated from each other when the reaction finished. The emulsification creates not only enormous interfacial area, but also very short distance for mass transfer. It should be mentioned that the baffles in the crucible contributes greatly in the mixing. Hence, the reaction can be considered to occur throughout the entire volume of the bath. On assumption that the whole volume of slag reacts with the whole volume of the silicon phase, and the reaction is controlled by first-order chemical reaction, the reaction rate can be expressed as
where k is the reaction rate constant. On integration, the aluminum concentration \( \left( {\text{mass pct Al}} \right)_{t} \) can be expressed as a function of time:
In Eq. [5], the final Al concentration, \( \left( {\text{mass pct Al}} \right)_{\text{final}} \)is the thermodynamic equilibrium concentration of Al in silicon. Note that it takes a few seconds to initiate the emulsification. This period is expected to be about 15 seconds. Hence, the zero-time is taken as the moment when the rotation motor has been engaged for 15 seconds. If the assumption that the process is controlled by chemical reaction is valid, all the experiments should have the same value of k in Eq. [5]. Based on the experimental data, the k value is evaluated for each plot in Figures 4 and 5. All the values are found to be similar. An average value is calculated to be k = 0.0248. The term coefficient of determination (R 2) is used to describe the analytical error. It ranges from 0.99 to 0.88 and the average value is 0.96. The k value is employed to predict the reaction curves. The model predictions are also included in Figures 4 and 5. It is seen in these two figures that the simple model can reproduce the reaction curves for all the cases satisfactorily. The fact that k does not depend on both the amount of slag and the initial composition of the slag is an evident confirmation to the assumption of chemical reaction control. If the reaction were mass transfer controlled, the reaction rate (consequently the rate constant) would have shown dependency on the amount of slag phase, since the convection would be directly related to the mass of the slag. Also, a mass transfer control (or partial control) would have shown the dependence of the reaction rate on the initial concentration of the slag, as the chemical gradient would be the driving force for mass transfer. The present simple model could be easily applied to a real ladle, if it is agitated efficiently by mechanical stirring.
As reported in the literature,[1–3] the impurity removal process is very slow without stirring, requiring 2-3 hours to reach slag-metal equilibrium. As shown by the present results, proper mechanical stirring would enhance greatly the removing rate of the impurities. In the laboratory experiments, silicon has reached equilibrium with the slag phase in only 300 seconds. The present results are also in good accordance with the finding in the studies on removal of B and Ca.[7,8] White and his coworker report that slag and silicon reach equilibrium very fast, within 300-600 seconds. Although a real industrial practice has many factors to consider, the present result still show evidently that mechanical stirring is a promising way to improve the silicon refining process by better utilization of the refining capacity of the slag phase.
Equilibrium Between the Slag and Liquid Silicon
As mentioned in the introduction, the thermodynamic data of the slag and liquid silicon hold the key to process development and optimization. One of the focus of the present work is to examine the applicability of the thermodynamic data, with respect to the slag-metal equilibrium.
Figures 4 and 5 shows evidently that the slag and metal have reached equilibrium after 600 seconds. It would be valuable to compare the experimental results with the model calculation. According to Turkdogan,[14] the standard Gibbs energy change for Reaction [1] is described as
The standard states for the species in Reaction [1] are pure solid Al2O3, pure solid SiO2, pure liquid aluminum and pure liquid silicon, respectively. The equilibrium constant K 1 is calculated to be 1374 at 1823 K (1550 °C) using Eq. [6]. Based on the slag compositions at equilibrium obtained by AAS, the activities of Al2O3 (\( a_{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} \)) and SiO2 (\( a_{{{\text{SiO}}_{ 2} }} \)) can be calculated using Factsage[15] or THERMO-Calc.[16] The activity of Si is very close to unity. Hence, the concentration of Al in silicon can be calculated using the thermodynamic equation for the activity coefficient of Al found in the literature based on Eq. [2].[10–13] The calculated Al concentrations using these equations are listed in Tables II, III compared with the experimentally determined values. Unfortunately, the calculated values in general do not agree with the experimental data. This is especially true in the case of low Al content. While the calculated values in the case of higher Al concentration shows somewhat better agreement using the equations by Dumay et.al.,[13] at low Al concentration, all calculated values are far from satisfactory. It implies that either the models for silicon phase or the slag model is incorrect. It is even possible that both the models for slag and liquid silicon need further improvement. It should be pointed out that the comparisons in Tables II and III cannot be used to examine the reliability of any of the individual models. The comparison can only show that the use of both the slag model and silicon model does not predict the slag-metal equilibrium. What model(s) need improvement would need further rigorous experimental studies.
As pointed out by Kero et al.,[9] the removing rate of element is correlated to the equilibrium distribution of the element between slag and metal. Hence, reliable models for both liquid silicon and slag would be essential for any process design and optimization.
Summary
To gain a better understanding of the removal of aluminum from silicon melt in silicon refining, experiments were carried out to study the kinetics of the process with mechanical stirring. The fact that the reaction rate was independent of the amount of slag and its composition strongly suggested that the reaction between silicon melt and slag (CaO-SiO2-Al2O3) is controlled by chemical reaction under the present experimental conditions. A simple mathematical model assuming chemical control could predict the reaction curves satisfactorily. A reaction rate constant i = 0.0248 was found to be applicable to all experiments at 1823 K (1550 °C). The silicon melt was in equilibrium with the slag phase after stirring for about 300 seconds. The experimentally obtained equilibrium concentrations of Al in the silicon phase and Al2O3 in the slag phase suggest strongly the need for new thermodynamic measurements in the silicon phase and slag phase with respect to aluminum.
References
M.D. Johnston and M.Barati: Sol. Energy Mater. Sol. Cells, 2010, vol. 94, pp. 2085-2090.
H. Nishimoto, Y. Kang, T. Yoshikawa, and K. Morita: High Temp. Mater. Proc., 2012, vol. 31, pp. 471–77.
T. Weiss and K. Schwerdtfeger: Metall. Mater. Trans. B, 1994, vol. 25(4), pp. 497-504.
J.C. Fulton, N.J. Grant, and J. Chipman: Trans. AIME, 1953, vol. 197, pp. 185-190.
W.L. Daines and R.D. Pehlke: Trans. AIME, 1968, vol. 242 pp. 565-75.
W. Pan, M. Sano, M. Hirasawa, and K. Mori: ISIJ Int., 31 (1991), 358-365
J.F. White and D. Sichen: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 96–105.
J.F. White and D. Sichen: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 135-144.
I. Kero, M.K. Naess, and V. Andersen, G.M. Tranell: Metall. Mater. Trans. B, 2015, vol. 46(3), pp. 1186-1194.
T. Miki, K. Morita, and N. Sano: Metall. Mater. Trans. B, 1998, vol. 29B, pp. 1043-1049.
T. Miki, K. Morita, and N. Sano: Mater. Trans. Jpn. Inst. Met.,1999, vol. 40, pp. 1108-1116.
L. Ottem: Equilibria between Molten Silicon and SiO2-CaO-Al2O3 Slags at 1550 °C. SINTEF Report STF34 F93112, 1993, Norway
C. Dumay, C. Chatillon, and M. Allibert: J. Chim. Phys., 1997, vol. 94, pp. 971-977.
E.T. Turkdogan, Physical Chemistry of High Temperature Technology; Academic Press, New York, 1980, pp 5-24.
G. Eriksson and A.D. Pelton: Metall. Mater. Trans. B, 1993, vol. 24B, pp. 807-816.
J.O. Andersson, T. Helander, L. Höglund, P.F. Shi, and B. Sundman: CALPHAD, 2002, vol. 26, pp. 273-312.
Acknowledgment
The financial support of the Norwegian Research Council (Project No. 235159) is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 14, 2016.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lee, J., White, J.F., Hildal, K. et al. Study on the Kinetics of Aluminum Removal from Liquid Silicon to Slag with Mechanical Stirring. Metall Mater Trans B 47, 3511–3518 (2016). https://doi.org/10.1007/s11663-016-0768-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0768-9