Abstract
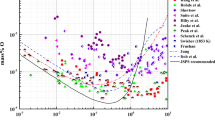
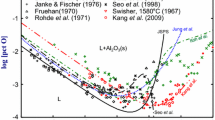
In order to provide accurate information for refining of steel containing more than 1 mass pct Al, previously known information about Al deoxidation equilibria in liquid iron was critically reviewed. New Al deoxidation equilibria, Al and O contents in liquid iron in equilibrium with solid Al2O3 were measured at 1873 K and 1923 K (1600 °C and 1650 °C) over the whole Al composition range, 0.0027 < [pct Al] < 100. In order to secure the deoxidation equilibria, in the present study, the Al deoxidation experiments were carried out by employing three different methods: (1) traditional Al deoxidation by the addition of Al into Fe-O alloys, (2) oxidation of Al in Fe-Al alloys by the addition of Fe2O3 as an oxygen source, and (3) addition of CaO flux for an effective removal of suspended Al2O3 inclusions in liquid alloys containing high Al. In addition, in the present study, the O solubility limit in pure Al melt in equilibrium with solid Al2O3 was also measured in the temperature range from 1673 K to 1873 K (1400 °C to 1600 °C). The present experimental results provide a complete set of Al deoxidation equilibria in liquid iron which may be useful for the estimation of residual oxygen level and alumina inclusion formation in high Al steel processing. Interaction parameter formalism, which was originally proposed by Wagner and Chipman and has been widely used to interpret the Al deoxidation equilibria in liquid iron, was found to be inapplicable. Limitation of the interaction parameter formalism at high Al content in liquid Fe was discussed.











Similar content being viewed by others
References
N. A. Gokcen and J. Chipman: J. Met., 1953, vol. 197, pp. 173-78.
A. McLean and H.B. Bell: J. Iron Steel Inst., 1965, vol. 203, p. 123-30.
J. H. Swisher: Trans. Metall. Soc. AIME, 1967, vol. 239, pp. 123–24.
R. J. Fruehan: Metall. Trans., 1970, vol. 1, pp, 3403-10.
L. E. Rohde, A. Choudhury, and M. Wahlster: Arch. Eisenhüttenwes., 1971, vol. 42, pp. 165-74.
D. Janke and W. A. Fischer: Arch. Eisenhüttenwes., 1976, vol. 47, 195-98.
V. E. Shevtsov: Russ. Metall., 1981, vol. 1, pp. 52-57.
H. Suito, H. Inoue, and R. Inoue: ISIJ Int., 1991, vol. 31, pp. 1381-88.
S. Dimitrov, A. Weyl, and D. Janke: Steel Res., 1995, vol. 66, pp. 3-7.
J. D. Seo, S.H. Kim, and K.R. Lee: Steel Res., 1998, vol. 69, pp. 49-53.
Y. J. Kang, M. Thunman, D. Sichen, T. Morohoshi, K. Mizukami, and K. Morita: ISIJ Int., 2009, vol. 49, pp. 1483-89.
G. R. St. Pierre: Metall. Trans. B, 1977, vol. 8B, pp. 215-7.
H. Itoh, M. Hino, and S. Banya: Tetsu-to-Hagané, 1997, vol. 83, pp. 773-78.
S. Gustafsson and P. O. Mellberg: Scand. J. Metallurgy, 1980, vol. 9, pp. 111-16.
D. Bouchard and C. W. Bale: J. Phase Equilib., 1995, vol. 16, pp. 16-23.
I. H. Jung, S. A. Decterov, and A. D. Pelton: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 493-507.
J. S. Kim, J. B. Jeon, J. E. Jung, K. K. Um, and Y. W. Chang: Met. Mater. Int., 2014, vol. 20, pp. 41-47.
B. H. Song, J. Kim, S. Jeong, I. Choi, and Y. K. Lee: Korean J. Met. Mater., 2014, vol. 52, pp. 1-9.
J. H. Park, D. J. Kim, and D. J. Min: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 2316-24.
C. H. P. Lupis and J. F. Elliott: Acta Metall., 1966, vol. 14, 529-38.
C. Wagner: Thermodynamics of Alloys, Addison-Wesley Press, Cambridge, MA, 1952, pp. 47-51.
J. Chipman: The Physical Chemistry of Liquid Steel, in Basic Open-Hearth Steel Making, The American Institute of Mining and Metallurgical Engineers, New York, 1951.
M.K. Paek, J.J. Pak, and Y.-B. Kang: Metall. Mater. Trans. B, 2015. DOI:10.1007/s11663-015-0369-z.
M.K. Paek, K.H. Do, Y.-B. Kang, I.H. Jung, and J.J. Pak: unpublished research.
R. Inoue and H. Suito: Mater. Trans. JIM, 1991, vol. 32, pp. 1164-9.
H. Yin: Proc. of Int. Conf. of AISTech 2005, vol. 2, Warrendale, PA, 2005, pp. 89–97.
The 19th Committee in Steelmaking: Thermodynamic Data For Steelmaking, The Japan Society for Promotion of Science, Tohoku University Press, Sendai, Japan, 2010, pp. 10–13.
L. S. Darken: Trans. AIME, 1967, vol. 239, pp. 80-89.
A. D. Pelton and C. W. Bale: Metall. Trans. A, 1986, vol. 17A, pp. 1211-5.
J. R. Taylor, A. T. Dinsdale, M. Hillert, and M. Selleby: CALPHAD, 1992, vol. 16, pp. 173-9.
A. D. Pelton: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 869-76.
A. D. Pelton and P. Chartrand: Metall. Mater. Trans. A, 2001, vol. 32A, pp. 1355-60.
Acknowledgment
This study was supported by the R&D Center for Valuable Recycling (Global-Top Environmental Technology Development Program) funded by the Ministry of Environment (Project No.: 11-C22-ID).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 19, 2014.
Rights and permissions
About this article
Cite this article
Paek, MK., Jang, JM., Kang, YB. et al. Aluminum Deoxidation Equilibria in Liquid Iron: Part I. Experimental. Metall Mater Trans B 46, 1826–1836 (2015). https://doi.org/10.1007/s11663-015-0368-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0368-0