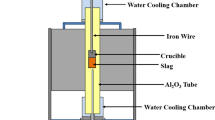
Abstract
In the present study, the applicability of the high-temperature mass spectrometric method combined with Knudsen effusion cell for quantifying the valence states of V in the multicomponent system CaO-MgO-Al2O3-SiO2-VO x up to a maximum temperature of 2050 K (1777 °C) was examined. The valence ratio of V3+/V4+ in slag phase was derived from the partial pressures of VO and VO2 in the effused vapor phase. The results show good agreement with the literature values obtained by other techniques. A correlation between the valence ratio V3+/V4+ and the oxygen partial pressure as well as basicity was achieved based on the present results and accessed data in the literature. The results of the present study demonstrate that the Knudsen cell-mass spectrometric method can be a very effective tool in estimating the valence ratios for of transition metals in metallurgical slags.





Similar content being viewed by others
References
V.L. Stolyarova and G.A. Semenov: Mass Spectrometric Study of the Vaporization of Oxide Systems, Wiley, Chichester.
L. J. Wang, S. Seetharaman: Metall. Mater. Trans. B, 2010, v41, no.5 pp.946-954.
H. J. Wang, V. L. Stolyarova, S. I. Lopatin, M.E. Kutuzova, S. Seetharaman: Rapid commun. Mass Spectrom. V.24, 16,2010, pp 2420-2430.
R. Mittelstädt, K. Schwerdtfeger: Metall. Trans. B, 1990, 21, pp.111-120.
R. Inoue, H. Suito: Trans. ISIJ, 22, 1982, pp.705-714.
A. Werme: Steel Res., 1988, 59(1), pp. 6-15.
H. Farah and M. Brungs: Journal of material science, 2003, vol. 38, pp.1885-1894.
H. Farah, Journal of material science, 2003, 38, pp.727-737.
H. Farah, M.P. Brungs, D.J. Miller and G.R. Belton: Physics and Chemistry of Glasses, 1998, 39(6), pp. 318-322.
P. L. Dong, X. D. Wang, S. Seetharaman: Steel Research International, 2009, 80(3), pp. 202-208.
W. Rammensee, H. Palme, and H. Wanke: Lunar and Planetary Sciences XIV, Houston, 1983, pp. 628–29.
G.A. Gaetani and T.L. Grove: Geochimica et Cosmochimica Acta, 61, 1997, pp.1829-1846.
B.Z. Hanson, J.H. Jones and G.A. Gaetani: EOS, 1996, 77, pp.846.
K.Righter S.R.Sutton, M. Newville, L.le, C.S Schwandt, H. Uchida, B. Lavina and R.T.Downs : American Mineralogist, 91, 2006, pp.1643-1656.
K.Righter S.R.Sutton,L.Danielson, K. Pando, G.Schmidt, H.Yang, S.serthet, M.Newville, Y. Choi, R.T.Downs and V.Malavergne: American Mineralogist, 96, 2011, pp.1278-1290.
S.B.Simon, S. R. Sutton and L. Grossman: Geochim. Cosmochim. Acta. 71, 2007, pp.3098-3118.
W.D. Johnston: Jour Amer. Ceramic Soc. 48, 1964, pp.608-611.
M. J. Toplis and A.Corgne: Contributions to Mineralogy and petrology, 144, 2002, pp.22-47.
H.D. Schreiber, R.C. Merkel, V.L. Schreiber, and G.B. Balazs: J. Geophys. Res. 92(B9), 1987, pp. 9233–45.
W. Banchorndhevakul, T. Matsui, and K. Naito: J. Nucl. Sci. Tech. 1986, 23(10), pp 873–82.
Acknowledgments
Financial support for this study was received from the National Nature Science Foundation of China (Nos. 51104013, 51174022), and the Swedish Foundation for Strategic Environmental Research (MISTRA) via Swedish Steel Producers Association (Jernkontoret) under the Eco steel Production program. The supports from these agencies are gratefully acknowledged. The authors express their sincere thanks to Dr. Haijuan Wang for providing the high-temperature mass spectrometry data for the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 28, 2012.
Rights and permissions
About this article
Cite this article
Wang, L., Teng, L., Chou, KC. et al. Determination of Vanadium Valence State in CaO-MgO-Al2O3-SiO2 System By High-Temperature Mass Spectrometry. Metall Mater Trans B 44, 948–953 (2013). https://doi.org/10.1007/s11663-013-9836-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9836-6