Abstract
A mathematical model of interdendritic thermometallurgical strain (ITM) generated during dendritic solidification of steel alloys has been developed. The model consists of two main parts in which the first part represents the derivation of ITM strain based on the concept of thermal storage energy. Consequently, the second one represents the volume changes associated with dendritic solidification phenomena during the interdendritic coherent region in the mushy zone. Calculations for Fe-C binary alloys show that the straining criteria such as strain rate and accumulated strain are sensitive to the solidification behavior of different steel alloys. The results also point out that good predications of different phases, accurate alloy properties (especially thermal expansion coefficient), and precise enthalpy functions during dendritic solidification are extremely necessary. Also, the predications show that the dendrite coherency criterion is considered an essential parameter to define ITM strain of particularly long solidification interval alloys. Model predications of ITM strain are compared and discussed with other established theoretical approaches and previous modeling work.











Similar content being viewed by others
Abbreviations
- A :
-
total elementary area of volume element in plane (m2)
- C eff :
-
equivalent heat capacity of CV (kJ/kgK)
- Cp :
-
heat capacity of CV (kJ/kgK)
- \( Cp_{\text{s}} \) :
-
heat capacity of solid phase (kJ/kgK)
- CV:
-
control volume element
- D γC :
-
diffusion coefficient of carbon in γ-phase (m2/s)
- E :
-
thermal storage energy (W/m3)
- \( \mathop E\limits^{ - } \) :
-
average energy capacity of CV (W/m3)
- E tr :
-
imparted heat conduction energy (W/m3)
- E in :
-
liberated inner heat energy (W/m3)
- E s :
-
solid enthalpy volumetric energy (W/m3)
- E L :
-
latent heat of fusion volumetric energy (W/m3)
- E p :
-
solid-state phase transformation volumetric energy (W/m3)
- E i :
-
volumetric energy resulting from change of phase i (W/m3)
- e wp :
-
first-order interaction coefficient of individual solutes in liquid iron
- f coh :
-
coherence factor
- f cry :
-
fraction of crystal
- f col :
-
fraction of columnar crystals
- f equ :
-
fraction of equiaxed crystals
- f s :
-
volume fraction of solid phase
- f is :
-
volume fraction of i-solid phase
- f i→js :
-
volume fraction of i-solid phase resulting from i → j solid phase transformation
- f L :
-
volume fraction of liquid phase
- f γ δ→γ :
-
volume fraction of austenite resulting from δ → γ solid phase transformation
- H i :
-
enthalpy of i dendritic phase (kJ/kg)
- \( \bar{H}_{\rm s} \) :
-
average enthalpy of solid phase (kJ/kg)
- H i→js :
-
enthalpy resulting from solid phase transformation from phase i → j (kJ/kg)
- i :
-
dendritic phase i
- N :
-
number of phases
- \( {}^{P}K_{\text{C}}^{i} \) :
-
partition coefficient of carbon in P-component system
- K mC :
-
binary partition coefficient for component m in iron
- q i :
-
heat flux in the direction i (kW/m2)
- Q ϕ , Q s :
-
heat flux at surface and solidus isotherm, respectively (kW/m2)
- Q coh :
-
heat flux at coherent isotherm (kW/m2)
- R d :
-
radius of primary dendrite arm spacing (μm)
- t :
-
time (s)
- T :
-
temperature (°C)
- T liq, T sol :
-
liquidus and solidus temperatures (°C)
- T coh, T pr :
-
coherence and peritectic temperatures (°C)
- V(T):
-
specific volume at temperature T (cm3/mol)
- V(T coh):
-
specific volume at coherence temperature T coh (cm3/mol)
- V k m :
-
molar volume of k-solid phase (cm3/mol)
- V l m :
-
molar volume of liquid phase (cm3/mol)
- x,y,z :
-
Cartesian coordinates (m)
- X kC :
-
mole fraction of carbon at k-phase
- X γ/δC :
-
mole fraction of carbon at γ/δ interface
- X γ/lC :
-
mole fraction of carbon at γ/l interface
- X lC :
-
mole fraction of carbon at liquid-phase
- α :
-
thermal expansion coefficient (1/K)
- χ l w :
-
concentration of element w in the liquid phase
- χ kC :
-
concentration of element carbon in the k-solid phase
- \( \varepsilon \) :
-
strain
- ɛ acc :
-
accumulative strain
- ɛ e :
-
elastic strain
- ɛ T :
-
thermal strain
- ɛ p :
-
plastic strain
- ɛ y–y :
-
total strain in direction y–y
- \( \dot{\varepsilon }_{c} \) :
-
strain rate of constant thermophysical property
- \( \dot{\varepsilon }_{v} \) :
-
strain rate of varied thermophysical property
- ∈ ph :
-
volumetric expansion strain
- λ :
-
thermal conductivity of a CV (W/mK)
- λ i :
-
thermal conductivity of phase I (W/mK)
- λ col :
-
equivalent thermal conductivity of a columnar structure (W/mK)
- λ equ :
-
equivalent thermal conductivity of a equiaxed structure (W/mK)
- λ L :
-
equivalent thermal conductivity of a liquid phase (W/mK)
- λ is :
-
equivalent thermal conductivity of i-solid phase (W/mK)
- λ 1 :
-
primary dendrite arm spacing (μm)
- ρ :
-
density (kg/m3)
- ρ L :
-
equivalent density of liquid phase (kg/m3)
- ρ s :
-
equivalent density of solid phase (kg/m3)
- ρ ks :
-
equivalent density of k-solid phase (kg/m3)
- ρ(T):
-
equivalent density of a control element at T (kg/m3)
- ρ(T coh):
-
equivalent density of a control element atT coh (kg/m3)
- ΔH L :
-
latent heat of fusion (kJ/kg)
- ΔH p :
-
latent heat of solid phase transformation (kJ/kg)
- \( \Updelta \dot{\varepsilon }_{T} \) :
-
difference strain rate at temperature T
References
J.K. Brimacombe and K. Sorimachi: Metall. Trans. B., 1977, vol. 8B, pp. 489-505.
M.C. Flemings: Metall. Trans. A, 1991, vol. 22A, pp. 957-81.
M. El-Bealy and H. Fredriksson: Metall. Mater. Trans., B, 1996, vol. 27B, pp. 999-1014.
M. El-Bealy: Metall. Mater. Trans., B, 2000, vol. 31B, pp. 331, 345.
Y.F. Guven and J.D. Hunt: Cast Met., 1988, vol. 1, pp. 104-11.
M. Rappaz, J.M. Drezet, and M. Gremaud: Metall. Mater. Trans. A, 1999, vol. 30A, pp. 449-56.
A. Grill, K. Sorimachi, and J.K. Brimacombe: Metall. Trans. B, 1976, vol. 7B, pp. 177-89.
J.O. Kristiansson: J. Therm. Stresses, 1982, vol. 7, pp. 209-24.
R.H. Tien and V. Loump: Trans. TMS-ASME, 1969, vol. 36, pp. 763-67.
M.O. El-Bealy: Proc. of TMS Conf., San Diego, CA, 2001.
K. Miyazaki, N. Kanamaru, N. Kaneko, and A. Ochi: Tetsu-to-Hagane, 1976, vol. 62, p. S482.
H. Fujii, T. Ohashi, and K. Hiromoto: Tetsu-to-Hagane, 1976, vol. 62, p. S484.
T. Matsumiya, M. Ito, H. Kajioka, S. Yamaguchi, and Y. Nakamura: ISIJ Int., 1986, vol. 26, pp. 540-46.
K. Marukawa, M. Kawasaki, T. Kimura, and S. Ishikawa: Tetsu-to-Hagane, 1978, vol. 64, p. S661.
Y. Sugitani, M. Nakamura, H. Kawashima, K. Kanazawa, H. Tomono, and M. Hashio: Tetsu-to-Hagane, 1982, vol. 68, p. A149.
K. Wünnenberg and R. Flender: Ironmaking Steelmaking, 1985, vol. 12, pp. 22-29.
S. Nagata, T. Matsumiya, K. Ozawa, and T. Ohash: Tetsu-to-Hagane, 1990, vol. 76, pp. 214-21.
A. Yamanaka, K. Nakajima, and K. Okamura: Ironmaking Steelmaking, 1995, vol. 22, pp. 508-12.
B.G. Thomas, I.V. Samaraskera, and J.K. Brimacombe: ISS Trans., 1986, vol. 7, p. 21.
K. Sorimachi and J.K. Brimacombe: Ironmaking Steelmaking, 1977, vol. 4, pp. 240-45.
W.S. Pellini: Foundry, 1952, vol. 80, pp. 125-33, 192-9.
T.W. Clyne and G.J. Davies: Br. Foundrymen, 1981, vol. 74, pp. 65-73.
U. Feurer: GieBerei-Forchung 1976, vol. 28, pp. 75-80.
J.-M Drezet and M. Rappaz: 1st ESAFORM Conf., on Material Forming, Eds. J.L. Chennot, J.F. Agassant, P. Montmitonnet, B. Vergnes, and N. Billon, EASFORM, Sophia Antipolis, France, 1998, pp. 49-57.
J.-M Drezet and M. Rappaz: Modelling of Casting, Welding and Advanced Solidification Processes-VIII, Eds. B.G. Thomas and C. Beckermann, TMS, San Diego, CA, 1998, pp. 883-89.
D.R. Poirier and P.J. Nandapurkar: Metall. Trans. A., 1988, vol. 19A, pp. 3057-61.
A. Jablonka, K. Harste, and K. Schwerdrfeger: Steel Res, 1991, vol. 62, pp. 24-33.
P.J. Wray: Metall. Trans. A., 1982, vol. 13A, pp. 125-34.
H. Fredriksson and J. Stjerndahl: J. Mater. Sci., 1982, vol. 16, pp. 575-85.
R.D. Pehlke, B.G. Thomas, and H. Wada: NTIS-PB83-21003, Technical Report, University of Michigan, Ann Arbor, MI, 1982.
J. Miettinen: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 281-97.
D.B. Spencer, R. Mehrabian, and M.C. Flemings: Metall. Trans., 1972, vol. 3, pp. 1925-32.
R.J. Claxton: J. Met., 1975, vol. 2, pp. 14-16.
G. Chai, L. Backnart, T. Rϕlland, and L. Arnberg: Metall. Mater. Trans. A., 1995, vol. 26A, pp. 965-70.
M.M Hamdi, S. Benum, D. Morttensen, H.G.FJ/ER, and J.–M. Drezet: Metall. Mater. Trans. A., 2003, vol. 34A, pp. 1941–52.
H. Fjaer, D.E.K. Jensen, and A. Mo: Proc. 5 th Int. Aluminum Extrusion Technology Seminar, Eds. R.I. Werner and V.R. Bird, The Aluminum Association, Aluminum Extruders Council, Wauconda, IL, 1992, p. 113.
M. El-Bealy and B.G. Thomas: Proc. of 54 th Electric Furnace Conf., Dallas, TX, 1996, pp. 565–79.
B.G. Thomas, I.V. Samaraskera, and J.K. Brimacombe: Metall. Trans. B., 1988, vol. 19B, pp. 277-87.
J.A. Spittle and A.A. Cushway: Met. Tech., 1983, vol. 10, pp. 6-13.
G. Van Drunen, J.K. Brimacombe, and F. Weinberg: Ironmaking Steelmaking, 1975, vol. 2, p. 125-33.
J.K. Brimacombe, F. Weinberg, and E.B. Hawbolt: Metall. Trans. B, 1979, vol. 10B, pp. 279-92.
D.R. Poirier, P.J. Nandapurkar, and S. Ganesan: Metall. Trans. B., 1991, vol. 22B, pp. 889-900.
Y. Ueshima, S. Mizoguchi, T. Matsumiya, and H. Kajioka: Metall. Trans. B., 1986, vol. 17B, pp. 845-59.
A. Mo and H. Thevik: Metall. Mater. Trans. A., 1998, vol. 29A, pp. 2189-94.
B. Rogberg: Ph.D. Dissertation, Royal Institute of Technology, Stockholm, Sweden, 1982.
H. Sandberg: Scand. J. Metall., 1973, vol. 2, pp. 233-41.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 124-129.
Y.S. Touloukian, R.W. Powell, C.Y. Ho, and P.G. Klemens: Thermal Conductivity, Metallic Elements and Alloys, vol. I, IFI/Plenum, New York, NY, 1970, pp. 652, 839.
M. El-Bealy and B.G. Thomas: Metall. Mater. Trans. B., 1996, vol. 27B, pp. 689-93.
H. Fredriksson: Met. Sci., 1976, vol. 10, pp. 77-86.
Acknowledgments
The author is especially grateful for the financial support from the Companies’ Chair of the Swedish Iron Masters Association, Stockholm, Sweden. He is gratefully indebted to Professor David Poirier, Department of Materials Science and Engineering, University of Arizona, for his endless encouragement and help, both professionally and privately, which has made it possible to finish this work. Also, the author’s sincere gratitude goes to Head of Tabbin Institute for Metallurgical Studies, Tabbin, Helwan, Egypt, and to the director and workers at its library.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 4, 2009.
Appendices
Appendix A. Thermal Contraction of Dendritic Solidification Element CV
Research showed that few direct data were available for the thermometallurgical contraction. The only relevant work has been produced by Jablonka et al.[27] As suggested by Jablonka et al.,[27] the thermometallurgical contraction of a dendritic solid is a function of the average density of CV. It is assumed that the average density of CV follows the mixture linear rule. Therefore, the density of a phase mixture containing liquid and various phases can be defined as follows:
According to Jablonka et al.[27] the thermometallurgical contraction can be expressed as follows:
where
and
By substituting Eqs. [A3] and [A4] into Eq. [A2], the thermometallurgical contraction can be rewritten as follows:
For carbon steels, the densities and specific volumes of different phases are illustrated in References 30 and 31 where they depend on temperature and carbon content. The values of these functions were tabulated in Table AI.
Appendix B. Liquid and Solid Phases of Dendritic Solidification Element CV
The solidification behaviors of carbon steels can be classified into four modes as shown in Figure 3, and the interdendritic and dendritic different phases can be calculated by Fredriksson’s approach.[29] Only a few explanations are provided here, and the reader is referred to the original references for the details, the assumptions of derivation, properties of steels and nomenclature made in the derivation. The liquid fraction f Lcan be computed as follows:
whereas the fraction of primary delta f δs for the first three modes as well as the fraction of primary gamma f γs for the fourth mode are determined by applying the following approach developed by BoRoberg[45]:
Equation [B2] was employed until the end of solidification for the first and fourth modes and until the peritectic temperature T pr for the second and third modes (Figure 3). The partition coefficient of carbon in the P-component system \( {}^{P}K_{\text{C}}^{i} \) can be approximated using the following relationship[46]:
The values of Eq. [B3] used in the computations are illustrated in Table AI.[46] The volume fraction of austenite f γs during δ → γ solid phase transformation in mode 1 (Figure 3(a)) can be calculated as follows:
In the second mode and at the peritectic temperature (Figure 3(b)), the previous approach was used and was reformulated as follows:
At the peritectic temperature in third mode, f γ l→γ was calculated using Eq. [B2], whereas f γ δ→γ was determined by applying the previous approach (Figure 3(c)), and the following equation was employed:
The mole fractions of carbon for different phasesX kC are taken from the equilibrium phase diagrams.[29,31] The diffusion coefficient D γC was assumed to be temperature dependent,[13] whereas molar volumes of austenite V γ m , ferrite V δ m , and liquid V l m were assumed to be a function of both temperature and carbon content. These functions are tabulated in Table AI.[28–31]
Appendix C. Thermal Conductivity for Dendritic Solidification element CV of Iron–Carbon Alloys
The effect of the thermal conductivity of CV λ is controlled by two main factors. The first factor is the type of crystal structure, whereas the second factor is different phases and their fractions.[19] Here, it is convenient for this problem to replace the effective thermal conductivity λ of CV in Eq. [13] with equal electrical resistances. Therefore, the type of crystal structure plays a major role in defining the heat pass model. For columnar crystals, the heat pass model follows two parallel resistances of dendritic solid and interdendritic liquid such as dendritic solidification or solid phase transformation and is expressed as follows:
For equiaxed crystals, the heat pass model can be represented as two resistances in series as follows:
However, for a mixture between columnar and equiaxed in CV, such as columnar equiaxed transition, the thermal conductivity of solidified alloys can formulated as follows:
The values of steel thermal conductivities used in Eqs. [C1] to [C3] depend on the temperature and carbon concentration in both the interdendritic liquid and the dendritic solid. The values of the thermal conductivity of different carbon steel alloys used in these computations are summarized in Table AI.[30,47,48]
Appendix D. Dendrite Coherency for Dendritic Solidification element CV of Iron–Carbon Alloys
During dendritic solidification, the dendrite coherency is an important parameter to define the coherent region in the mushy zone and, therefore, to calculate the interdendritic strain accurately.[34] This criterion depends on the dendritic growth rate V a and primary dendrite arm radius R d where the V a /R d ratio is inversely proportional to the coherency fraction solid in the alloy. The same equation proposed by Chai et al.[34] was employed and can be computed as follows:
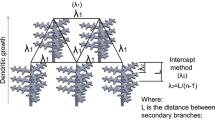
where a and b are constants and equal to 0.8269 and –0.1091, respectively, whereas R d is equal to half of primary dendrite arm spacing \( {\frac{{\lambda_{1} }}{2}} \) as shown in Figure 2(b) and it can be calculated using the same approach as El-Bealy and Thomas in Reference 49.
Equation [D1] was used to calculate f coh in the primary delta f δs region shown in Figures 3(a) through (c) for carbon steel alloys. But for the peritectic reaction steel alloy (Figures 3(b) and (c)), and if f coh is greater than f per, the following equation can be used:
This is because of the nature of the fast solidification process and the solid phase transformation rates of peritectic steels at peritectic temperature.[50]
Rights and permissions
About this article
Cite this article
El-Bealy, M.O. A Mathematical Model of Interdendritic Thermometallurgical Strain for Dendritic Solidification Processes. Metall Mater Trans B 42, 1280–1296 (2011). https://doi.org/10.1007/s11663-010-9464-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9464-3