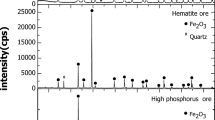
Abstract
The reduction of iron oxide fines to wustite between 590 °C and 1000 °C with a CO–CO2 gas mixture of low reducing potential was studied. The reduction kinetics and the dominating reaction mechanism varied with the temperature, extent of reduction, and type of iron oxide. Reduction from hematite to wustite proceeded in two consecutive reaction steps with magnetite as an intermediate oxide. The first reduction step (hematite to magnetite) was fast and controlled by external gas mass transfer independently of the oxide type and the temperature employed. The second reduction step (magnetite to wustite) was the overall reaction-controlling step, and the reduction mechanism varied with the temperature and the oxide type. Moderately porous oxide fines followed the uniform internal reaction for the temperature range studied. For highly porous oxides, the second reduction step was controlled by external gas mass transfer above 700 °C. Below that temperature, a mixed regime that involves external gas mass transfer and limited mixed control, which comprises pore diffusion and chemical reaction, takes place. The rate equations for this mixed control reaction mechanism were developed, and the limited mixed control rate constant (klm) was computed. For denser oxides under uniform internal reaction, the product of the rate constant and pore surface area (k·S) was calculated.











Similar content being viewed by others
References
E.T. Turkdogan and J.V. Vinters: Metall. Trans., 1971 vol. 2, pp. 3175-88.
Turkdogan, E.T.: Metall. Trans. B, 1978, vol. 9, pp. 163-79.
R. Corbari and R.J. Fruehan: Carnegie Mellon University, unpublished research, 2004.
R.J. Fruehan, K. Ito, and B. Ozturk: Trans. ISS: 1988, pp. 83–91.
R.J. Fruehan: Trans. ISS, 2003, vol. 30 no. 2, pp. 48-60.
R.S. Sampaio, R.J. Fruehan and B. Ozturk: Trans. ISS, 1993, vol. 14, pp. 59-67.
E.T. Turkdogan: Metall. Mater. Trans. B, 1978, vol. 9, pp. 163-79.
A.A. El-Geassy and M.I. Nasr: Iron Steel Inst. Jpn., 1988, vol. 28, no. 8, pp. 650-8.
H.Y. Sohn and J. Szekely: Chem. Eng. Sci., 1972, vol. 27, no. 4, pp. 763.
S.P. Trushenski, K. Li and W.O. Philbrook: Metall. Trans., 1974, vol. 5, pp. 1149-58.
J. Szekely, J.W. Evans and H.Y. Sohn: Heterogeneous Reaction Kinetics, Academic Press, Washington, DC, 1976.
J. Szekely and J.W. Evans: Metall. Trans., 1971, vol. 2, pp. 1691-711.
E.T. Turkdogan and J.V. Vinters: Metall. Trans., 1972, vol. 3, pp. 1561-74.
R.H. Spitzer, F.S. Manning and W. Philbrook: Trans. Metall. Soc. AIME, 1966, vol. 236, no. 5, pp. 726.
W.M. Mckewan: Trans. Am. Inst. Mining Metall. Eng., 1958, vol. 212, pp. 791–93.
R.H. Tien and E.T. Turkdogan: Metall. Trans., 1972, vol. 3, pp. 2039-48.
S.R. Story and R.J. Fruehan: Metall. Mater. Trans. B, 2000, vol. 31, pp. 43-54.
R.W. Pollard and R.D. Present: Phys. Rev., 1948, vol. 73, pp. 762-74.
G.R. Youngquist: Flow Through Porous Media, American Chemical Society, Washington, DC, 1970
A. Wheeler: Reaction Rates and Selectivity in Catalyst Pores, in Advances in Catalysis and Related Subjects, Academic Press, Washington, DC, 1951.
T. Murayama, Y. Ono and Y. Kawai: Iron Steel Inst. Jpn., 1977, vol. 63, no. 7, pp. 1099-107.
R.D. Doherty, K.M. Hutchings, J.D. Smith and S. Yörük: Metall. Mater. Trans. B, 1985, vol. 16, pp. 425-32.
H.Y. Sohn: Chem. Eng. Sci., 2004, vol. 59, pp. 4361-68.
K. Piotrowski, K. Mondal, T. Wiltowski, P. Dydo and G. Rizeg: Chem. Eng. J., 2007, vol. 131, no. 1-3, pp. 73-82.
T. Ariyama, S. Isozaki, S Matsubara, H. Kawata, K. Kondo and I. Kobayashi: J. Iron Steel Inst. Jpn., 1993, vol. 79, no. 12, pp. 1323-28.
N. Towhidi and J. Szekely: Metall. Trans. B, 1983, vol. 14, pp. 359-67.
D. Wagner, O. Devisme, F. Patisson, and D. Ablitzer: Sohn International Symp., San Diego, CA, 2006.
N.A. Warner: Trans. Metall. Soc. AIME, 1964, vol. 230, pp. 163-76.
R. Corbari: Ph.D. Thesis, Carnegie Mellon, Pittsburgh, PA, 2008.
P.B. Weisz and A.B. Schwartz: J. Catal., 1962, vol. 1, pp. 399-406.
R.M. German: Sintering Theory and Practice, Wiley, New York, NY, 1996.
Acknowledgments
The authors wish to thank the member companies of the Center for Iron and Steelmaking Research for the funding of this research. Iron oxide samples provided by Vale and the U.S. Steel Technology Center as well as laboratory analysis by Vesuvius Research also are appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 8, 2009.
APPENDIX: External Gas Mass Transfer Determination
APPENDIX: External Gas Mass Transfer Determination
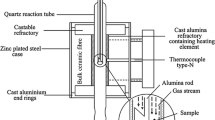
The mass transfer coefficient of CO–CO2 (mCO-CO2) that was used to compute the reduction rate associated with external gas transfer was determined indirectly by measuring the evaporation rate of Mg under Argon-5 pct H2 and H2 at 625 °C. The equipment, flow and geometric conditions of the evaporation experiments were identical to those conditions of the iron oxide reduction. The results are shown in Figure A1.
The evaporation rate of magnesium in Argon (RMg-Ar), which is controlled by external gas phase mass transfer, is expressed in Eq. [A1], where mMg-Ar is the mass transfer coefficient of Mg in Argon, and p SMg and p BMg represent the partial pressure of Mg in the surface and bulk stream, respectively.
Using Eq. A1, the mass transfer coefficient (m Mg-Ar) is calculated with the experimental evaporation rate and by taking Aδ as the crucible area; \( p_{\rm Mg}^{S} \) is calculated as the equilibrium vapor pressure of Mg, whereas \( p_{\rm Mg}^{B} \) is assumed to be negligibly small. The mass transfer coefficient of Mg–Ar at 625 °C then is extrapolated to the temperature range of interest (600 °C–1000 °C) and converted to mass transfer of CO–CO2 which accounts for the differences in gas diffusivities. This methodology was validated using the Mg–H2 evaporation results also shown in Figure A1.
Rights and permissions
About this article
Cite this article
Corbari, R., Fruehan, R. Reduction of Iron Oxide Fines to Wustite with CO/CO2 Gas of Low Reducing Potential. Metall Mater Trans B 41, 318–329 (2010). https://doi.org/10.1007/s11663-009-9315-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-009-9315-2