Abstract
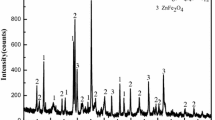
The possibility of metals recovery from zinc ferrite residues using transformational roasting processes was examined by roasting zinc ferrite residue from Doe Run Peru’s La Oroya plant (Peru), containing 19.5 pct Zn, 26.6 pct Fe, 750 g/t In, and 520 g/t Ga, with Na2CO3 and leaching with 200 g/L H2SO4 solutions. The X-ray diffraction (XRD) and diagnostic leaching tests indicate that approximately 87 pct of the zinc in this residue is present as franklinite (ZnFe2O4), with the remaining zinc present as entrained ZnSO4 or unleached ZnO. Both preliminary and design of experiments (DOE) testing, using a 22 central composite design (CCD), were performed to test the effects of temperature and a Na2CO3 addition on metals extraction and on the formation of minerals during roasting, and the solubility of these minerals during leaching. Both methods of testing showed that zinc and iron extractions increased with increasing temperature and Na2CO3 additions over the range of conditions tested. Roasting at 950 °C and 80 pct Na2CO3 produced a roasted residue from which 99 pct of the Zn, 88 pct In, and 85 pct Ga could be recovered by leaching, but from which up to 81 pct Fe was also dissolved. Mineralogical analysis using XRD and scanning electron microscopy (SEM)/energy dispersive X-ray (EDX) analysis showed that, for these conditions, ZnFe2O4 decomposes in the presence of Na2CO3 to form ZnO and either α-NaFeO2 or β-NaFeO2. Some of the ZnO formed reacts with Na2CO3 and silicates in the residue to form Na2ZnSiO4 and some unreacted Na2CO3/Na2O/Na2SO4 was also identified after roasting using SEM/EDX. All these phases are dissolved in acid leaching, leaving unreacted ZnFe2O4 and precipitated PbSO4 as the only phases identified in the leach residues. These results indicate that NaFeO2 is formed preferentially to Fe2O3 during roasting and that the NaFeO2 formed during roasting is highly soluble in acidic solutions. The results were also compared with studies on the roasting of more ZnFe2O4-deficient electric arc furnace (EAF) dusts with Na2CO3 or NaOH and indicated that, although roasting with Na2CO3 required higher roasting temperatures to achieve high zinc extractions, much lower Na2CO3 additions are required and higher indium recoveries are possible, if the combination of Na2CO3 roasting and H2SO4 leaching is used.









Similar content being viewed by others
Notes
FREED is a trademark of THERMART, San Diego, CA.
FACTSAGE is a trademark of Thermfact/CRCT, Montreal, and GTT-Technologies, Aachen, Germany.
DOE PRO XL is a trademark of Digital Computations, Inc., Orlando, FL, and Air Academy Associates, LLC, Colorado Springs, CO.
PERKIN ELMER is a trademark of Perkin Elmer, Inc., Wellesley, MA.
HITACHI is a trademark of Hitachi High-Technologies Canada, Inc., Rexdale, ON, Canada.
GW ELECTRONICS is a trademark of EBSciences, East Granby, CT.
PRINCETON GAMMA TECH is a trademark of Princeton Gamma Tech, Princeton, NJ.
RIGAKU is a trademark of Rigaku Americas Corporation, The Woodlands, TX.
JADE is a trademark of Materials Data, Incorporated, Livermore, CA.
References
D.W. Hopkins: Bull. Inst. Min. Metall., 1949, vol. 515, pp. 1–21
G.G. Graf: Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed., Wiley Interscience, New York, NY, 2005, pp. 1–23
G.A. Komlev, V.N. Gareev: Tsv. Met. (Moscow), 1964, vol. 37 (3), pp. 22–29
C.Y. Choi, Y.H. Lee: REWAS ‘99—Global Symp. on Recycling, Waste Treatment and Clean Technology, TMS, Warrendale, PA, 1999, pp. 1613–22
P.C. Holloway, T.H. Etsell: EPD Congress 2006, TMS, Warrendale, PA, 2006, pp. 525–34
J. Reyes: Doe Run Peru, La Oroya, Peru, private communication, 2004
J. Chadwick: J. Min. Mag., 1998, vol. 178 (2), pp. 106 and 108–11
M.B. Morsi, M.M. Nasr, F.H.A. Abdalla, S.Z. El-Tawil: Trans. Ind.n Inst. Met., 1998, vol. 51 (4), pp. 193–200
D.K. Xia, C.A. Pickles: Can. Metall. Q., 2000, vol. 38 (3), pp. 175–86
Z. Youcai, R. Stanforth: J. Haz. Mater., vol. B80, 2000, pp. 223–40
S. Okamoto: J. Solid State Chem., 1981, vol. 39, pp. 240–45
J.W. Kim, H.G. Lee: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 17–24
Acknowledgments
The authors would like to thank Doe Run Peru for supplying a sample of zinc ferrite residue for metallurgical testing. The authors also would like to thank the Natural Sciences and Engineering Research Council (NSERC) of Canada and Alberta Ingenuity for supplying student funding that helped support this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 10, 2007.
Rights and permissions
About this article
Cite this article
Holloway, P., Etsell, T. & Murland, A. Roasting of La Oroya Zinc Ferrite with Na2CO3 . Metall Mater Trans B 38, 781–791 (2007). https://doi.org/10.1007/s11663-007-9082-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-007-9082-x