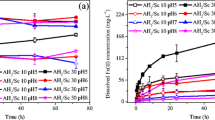
In this work, the hydrothermal synthesis and the chemical stability of several Fe (III)-Al (III) arsenate solid solution materials (Fe1−x Al x AsO4 · 2H2O) are described. Their synthesis involved hydrothermal precipitation at 160 °C from Fe (III)-Al (III)-As (V) nitrate solutions with fixed [As] = 0.3 M and ([Fe] + [Al]) = 0.33 M over a period of 24 hours. The produced solid solutions are compared to the two end members of the series, namely, scorodite (FeAsO4 · 2H2O) and mansfieldite (AlAsO4 · 2H2O). The members of the solid solution series were found to exhibit some differences in terms of particle size, shape, morphology, lattice parameters, and stability as a function of the Al fraction in the scorodite structure. It appears that materials with low Al content exhibit characteristics similar to scorodite, whereas materials with high Al content resemble mansfieldite. Extended leachability studies (up to 6 weeks) in the pH range 3 to 7 at 22 °C showed that their stability decreases with increasing Al fraction in their structure.











Similar content being viewed by others
References
W.T.A. Harrison: Acta Crystallogr. Sect. C: Cryst. Struct. Commun., 2000, 56, 421
R. Kniep, D. Mootz, A. Vegas: Acta Crystallogr. Sect. B: Struct. Sci, 1977, 33, 263–65
K. Kitahama, R. Kiriyama, Y. Bala: Acta Crystallogr. Sect. B: Struct. Sci, 1975, 31, 322–24
F.C. Hawthorne: Acta Crystallogr. Sect. B: Struct. Sci, 1976, 32, 2891–92
J.E. Dutrizac, J.L. Jambor: Hydrometallurgy, 1988, 19, 377–84
P.M. Swash, A.J. Monhemius: Hydrometallurgy ‘94, Chapman, & Hall, London, 1994, pp. 177–90
G.P. Demopoulos, D.J. Droppert, G. Van Weert: Hydrometallurgy, 1995, 38, 245–61
S. Singhania, Q. Wang, D. Filippou, G.P. Demopoulos: Metall. Mater. Trans. B, 2005, 36B, 327–33
S. Singhania, Q. Wang, D. Filippou, G.P. Demopoulos: Metall. Mater. Trans. B, 2006, 37B, 189–97
P.M. Dove, J.D. Rimstidt: Am. Mineral., 1985, 70, 838–44
E. Krause, V.A. Ettel: Am. Mineral., 1988, 73, 850–54
R.G. Robins: in EPD Congress’90, D.R. Gaskell, ed., TMS, Warrendale, PA, 1990, pp. 93–104
M.C. Bluteau and G.P. Demopoulos: Hydrometallurgy, 2007, (accepted)
D. Langmuir, J. Mahoney, J. Rowson: Geochim. Cosmochim. Acta, 2006, 70, 2942–56
G.P. Demopoulos: in Arsenic Metallurgy, R.G. Reddy, V. Ramachandran, eds., TMS, Warrendale, PA, 2005, pp. 25–50
V.T. Allen, J.J. Fahey, J.M. Axelrod: Am. Mineral., 1948, 33, 122–34
M. Ronis, F. d’Yvoire: Bull. Soc. Chim. Fr., 1974, 1–2, 78–82
J.F. Le Berre, T.C. Cheng, R. Gauvin, and G.P. Demopoulos: Can. Metall. Q., 2007, 46, 1–10
J.M. Rincon, M. Romero, A. Hidalgo, M.J. Liso: J. Therm. Anal. Calorim., 2004, 76, 903–11
F.W. Clarke: J. Wash. Acad. Sci, 1912, 2, 516–18
R.M. Denning: Am. Mineral., 1943, 28, 55–57
G. Winter: Fortsch. Miner., 1979, 57, 172–202
“Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; Methods 1311: Toxicity Characteristic Leaching Procedure,” Document SW-846, Environmental Protection Agency, 1992. http://www.epa.gov/epaoswer/hazwaste/test/pdfs/1311.pdf
Acknowledgments
The authors are grateful to Anne Fagot for her help with the stability experiments. Funding for this research was received through a NSERC Strategic Projects grant. The research was sponsored by Barrick Gold Corporation, Areva Resources, Hatch Ltd., and Placer Dome Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 19, 2006.
Rights and permissions
About this article
Cite this article
Le Berre, J., Cheng, T., Gauvin, R. et al. Hydrothermal Synthesis and Stability Evaluation of Iron (III)-Aluminum (III) Arsenate Solid Solutions. Metall Mater Trans B 38, 159–166 (2007). https://doi.org/10.1007/s11663-007-9032-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-007-9032-7