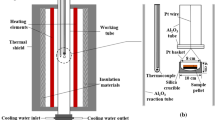
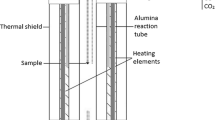
Abstract
Calcium ferrite slag has been successfully used in the copper smelting process, but no attempt has been made to use it in the nickel smelting process. The phase equilibrium and the distribution of minor elements between the Ni3S2-FeS matte and the CaO-FeOx-based slag (containing about 2 wt pct MgO) in a magnesia crucible were investigated at 1523 K under controlled partial pressures of S2, O2, and SO2 of 10.1, 50.7, and 101.3 kPa, respectively. The results were compared with those for the iron-silicate-based slag, and the following conclusions were obtained: (1) there is no significant difference in the solubility of nickel between both slags in the high-matte-grade range, (2) the dissolution of cobalt in the calcium ferrite slag is clearly smaller than that in the iron silicate slag, (3) detrimental arsenic, antimony, and bismuth are preferentially collected and fixed in the calcium ferrite slag rather than in the iron silicate slag, and (4) it is considered, with regard to technical feasibility, that the use of the calcium ferrite slag in a converting process of the Bessemer matte will have a prominent future for the nickel converting stage.
Similar content being viewed by others
Abbreviations
- P i :
-
partial pressure of species i, Pa
- p i :
-
potential of i (p i = P i/101, 325 Pa)
- K [n] :
-
equilibrium constant of reaction n
- a i :
-
activity of species i
- γ i :
-
activity coefficient of species i
- percent Me:
-
concentration of metal Me in the slag phase
- M Me :
-
atomic weight of metal Me
- n t :
-
total number of moles of the components in 100 g of either slag or matte phase
- ΔG o :
-
Gibbs free energy, J
- T :
-
temperature, K
- L s/mx :
-
distribution ratio between slag and matte phases for element X
- (), {}:
-
slag and matte phases, respectively
References
A.R. Burkin: Extractive Metallurgy of Nickel, John Wiley & Sons, New York, NY, 1987, p. 14.
J.M. Font, Y. Takeda, and K. Itagaki: Mater. Trans. Jpn. Inst. Met., JIM, 1998, vol. 39, pp. 652–57.
J.M. Font, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1998, vol. 39, pp. 834–40.
J.M. Font, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1999, vol. 40, pp. 20–26.
J.M. Font, M. Hino, and K. Itagaki: J. Min. Mater. Proc. Inst. Jpn., SHIGEN TO SOZAI, 1999, vol. 115(6), pp. 460–65.
A. Yazawa: Can. Metall. Q., 1974, vol. 13, pp. 443–53.
O. Knacke, O. Kubaschewski, and H. Hesselmann: Thermochemical Properties of Inorganic Substances, Springer-Verlag, Berlin, 1991, pp. 307, 309, 1438, 1455, 1459, 1493, 1709, and 1744.
Y. Takeda, S. Nakazawa, and A. Yazawa: Can. Metall. Q., 1980, vol. 19, pp. 297–305.
G. Roghani, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1997, vol. 38(8), pp. 707–13.
G.S. Victorovich, C. Diaz, and L. Timberg: Canadian Metallurgical Quarterly, 1985, vol. 24, pp. 363–68.
K. Hsieh and Y.A. Chang: Can. Met. Q., 1987, vol. 26, pp. 311–27.
G. Roghani, J.M. Font, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1996, vol. 37, pp. 1574–79.
A. Yazawa: Erzmetallurgy, 1980, vol. 33, pp. 377–82.
S.N. Sinha, H.Y. Sohn, and M. Nagamori: Metall. Trans. B, 1985, vol. 16B, pp. 53–59.
Y. Takeda, S. Ishiwata, and A. Yazawa: Mater. Trans. JIM, 1983, vol. 24(7), pp. 518–28.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Font, J.M., Hino, M. & Itagaki, K. Phase equilibrium and minor-element distribution between Ni3S2-FeS matte and calcium ferrite slag under high partial pressures of SO2 . Metall Mater Trans B 31, 1231–1239 (2000). https://doi.org/10.1007/s11663-000-0010-6
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-000-0010-6