Abstract
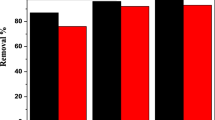
The extraction of concentrated iron(III) from acid chloride solutions has been investigated with methyl isobutyl ketone (MIBK), tri-n-butyl phosphate (TBP), di(2-ethylhexyl) phosphoric acid (D2EHPA), and their mixtures in various proportions, at different acid concentrations. On comparing the extraction of iron(III) with mixed and individual extractant, it was found that both D2EHPA-MIBK and D2EHPA-TBP mixtures exhibit synergism, the latter having better extraction ability. The synergistic coefficients, at different initial acid concentrations for each mixed extractant system, were evaluated and compared. An increase in the concentration of MIBK and TBP in the mixed organic resulted in higher synergistic coefficient. The stripping of iron(III) from loaded D2EHPA was found to increase with the strip feed acid concentration, while from loaded organic mixtures, it initially increased and then decreased. Stripping of iron(III) from D2EHPA-MIBK loaded solvent is better then D2EHPA-TBP. The extracted iron species formed and the stripping reactions have been proposed. Ultraviolet visible spectra of the stripped organic phase support the result and the proposed mechanisms.
Similar content being viewed by others
References
A. Chiba and O. Kimura: Proc. 5th Int. Conf. on Ferrites (ICF-5), Bombay, India, Jan. 10–13, 1989, C.M. Srivastava and M.J. Patni, eds., Oxford and IBH Publishing Co., New Delhi, 1989, p. 35.
E.C. Snelling: Soft Ferrites: Properties and Applications, 2nd ed., Butterworth and Co., London, 1988.
G.P. Demopoulos and D.L. Gefvert: Hydrometallugy, 1984, vol. 12, pp. 229–315.
F.J. Alguacil and S. Amer: Hydrometallurgy, 1986, vol. 15, pp. 337–50.
F. Islam, H. Rahman, and M. Ali: J. Inorg. Nucl. Chem., 1979, vol. 41, pp. 217–21.
J. Chen, S. Yu, H. Liu, X. Meng, and Z. Wu: Hydrometallurgy, 1992, vol. 30, pp. 401–15.
T. Sekine, H. Horida, and Y. Zeniya: J. Inorg. Nucl. Chem., 1976, vol. 38, pp. 1347–52.
J.W. Roddy, C.F. Coleman, and S. Arai: J. Inorg. Nucl. Chem., 1971, vol. 33, pp. 1099–1118.
F.J. Alguacil, S. Amer, and A. Luis: Hydrometallurgy, 1987, vol. 18, pp. 65–73.
M.I. Sytefanakis and A.J. Monhemius: Hydrometallurgy, 1985, vol. 15, pp. 113–39.
S.K. Majumdar and A.K. De: Talanta, 1960, vol. 7, pp. 1–6.
H. Irving and D.N. Edgington: J. Inorg. Nucl. Chem., 1959, vol. 10, pp. 306–18.
T. Sato, T. Nakamura, and M. Ikeno: Hydrometallurgy, 1985, vol. 15, pp. 209–17.
J.W. Roddy, C.F. Coleman, and S. Arai: J. Inorg. Nucl. Chem., 1971, vol. 33, p. 1099.
T. Hirato, W. Zhi-Chu, Y. Yamada, and H. Majima: Hydrometallurgy, 1992, vol. 28, pp. 81–93.
R.T. Kimura, P.A. Haunschild, and K.C. Liddell: Metall. Trans. B, 1984, 15B, pp. 213–19.
S. Agatzini, A. Kontopoulos, P. Maraboutles, and A. Xenidis: in Iron Control in Hydrometallurgy, J.E. Dutrizac and A.J. Monhemius, eds., Horwood, Chichester, United Kingdom, 1986, pp. 353–74.
K. Nagashima and I. Tomita: Analytical Chemistry, Shoukabou Publishing Co. Ltd., Tokyo, 1975, p. 192.
S. Yu and J. Chen: Proc. Int. Conf. Hydrometallurgy, Y. Zheng and J. Xu, eds., 1st International Academic Publishers, Beijing, 1988, pp. 185–88.
S. Yu and J. Chen: Hydrometallurgy, 1989, vol. 22, pp. 183–92.
H.L. Finston and Y. Inoue: J. Inorg. Nucl Chem., 1967, vol. 29, pp. 2431–40.
K.K. Sahu and R.P. Das: CHEMCON-95, IGCAR, Kalpakkam, India, Dec. 27–30, 1995.
K.K. Sahu and R.P. Das: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 181–89.
A.I. Vogel: A Textbook of Quantitative Inorganic Analysis, 4th ed., ELBS, Longman Inc., New York, NY, 1978, p. 399.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sahu, K.K., Das, R.P. Mixed solvent systems for the extraction and stripping of iron(III) from concentrated acid chloride solutions. Metall Mater Trans B 31, 1169–1174 (2000). https://doi.org/10.1007/s11663-000-0003-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-000-0003-5